Difference between revisions of "Hemocyanin" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 5: | Line 5: | ||
== Structure and mechanism == | == Structure and mechanism == | ||
| + | Hemocyanins use copper-binding sites to bind and transport oxygen. Hemocyanins typically have high molecular weights and are made of several individual subunit proteins, with each subunit containing two [[copper]] atoms and able to bind one oxygen molecule (O<sub>2</sub>). The subunits tend to aggregate. Oxygen affinity is affected by pH, temperature, and ionic concentration (Nigam et al. 1997). | ||
| − | + | The structure of arthropod hemocyanin tends to be quite different from that of mollusks (Nigam et al. 1997). | |
| − | + | In [[arthropod]]s, hemocyanin is composed of six subunits, or multiples of six subunits (Nigam et al. 1997). Such is the case, for example, in [[crayfish]], [[lobster]]s, and [[crab]]s, where the structures are hexameric or dodecameric (protein complex with 12 protein subunits) (Nigam et al. 1997). Each subunit weighs about 75,000 daltons (75 kDa) and has two copper atoms. The subunits each have about three domains with oxygen bound in the second domain (Nigam et al. 1997). Each subunit of two copper atoms binds one molecule of O<sub>2</sub>. | |
| − | + | In [[mollusk]]s, the hemocyanin is about 290,000 daltons (290 kDa), with two copper units for each 50,000 daltons (Nigam et al. 1997). The polypeptide chain binds about six to eight O<sub>2</sub> molecules (Nigam et al. 1997). | |
| − | |||
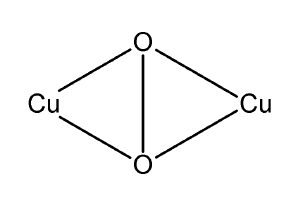
[[Image:Oxyhemocyanin.png|thumb|right|Oxygen binding mode with respect to copper centers]] | [[Image:Oxyhemocyanin.png|thumb|right|Oxygen binding mode with respect to copper centers]] | ||
| − | Spectroscopy of oxyhemocyanin shows several salient features: | + | Spectroscopy of oxyhemocyanin shows several salient features: |
# resonance [[Raman spectroscopy]] shows symmetric binding | # resonance [[Raman spectroscopy]] shows symmetric binding | ||
| − | # [[UV-Vis spectroscopy]] shows strong | + | # [[UV-Vis spectroscopy]] shows strong absorbencies at 350 and 580 nm. |
# OxyHc is [[electron paramagnetic resonance|EPR]]-silent indicating the absence of unpaired electrons | # OxyHc is [[electron paramagnetic resonance|EPR]]-silent indicating the absence of unpaired electrons | ||
# [[Infrared spectroscopy]] shows ν(O-O) of 755 cm<sup>-1</sup> | # [[Infrared spectroscopy]] shows ν(O-O) of 755 cm<sup>-1</sup> | ||
| − | + | Feature one rules out a mononuclear peroxo complex. Feature two does not match with the UV-Vis spectra of mononuclear peroxo and [[Kenneth Karlin]]'s trans-peroxo models (Karlin et al. 1987). Feature four shows a considerably weaker O-O bond compared with Karlin's trans-peroxo model (Karlin et al. 1987). On the other hand, [[Nobumasa Kitajima]]'s model shows ν(O-O) of 741 cm<sup>-1</sup> and UV-Vis absorbencies at 349 and 551 nm, which agree with the experimental observations for oxyHc (Kitajima et al. 1992). The weak O-O bond of oxyhemocyanin is because of metal-ligand backdonation into the σ<sup>*</sup> orbitals. The donation of electrons into the O-O antibonding orbitals weakens the O-O bond, giving a lower than expected infrared stretching frequency. | |
| − | |||
| − | |||
| − | + | ==Hemocyanin versus hemoglobin== | |
| − | + | Although the respiratory function of hemocyanin is similar to that of [[hemoglobin]], there are a significant number of differences in its molecular structure and mechanism. Whereas hemoglobin carries its [[iron]] atoms in [[porphyrin]] rings ([[heme]] groups), the [[copper]] atoms of hemocyanin are bound as [[prosthetic group]]s coordinated by [[histidine]] residues. Species using hemocyanin for oxygen transportation are commonly [[crustaceans]] living in cold environments with low oxygen pressure. Under these circumstances hemoglobin oxygen transportation is less efficient than hemocyanin oxygen transportation. | |
| + | |||
| + | Most hemocyanins bind with oxygen non-cooperatively and are roughly one-fourth as efficient as hemoglobin at transporting oxygen per amount of blood. Hemoglobin binds oxygen cooperatively due to steric [[protein folding|conformation]] changes in the [[protein complex]], which increases hemoglobin's affinity for oxygen when partially oxygenated. In some hemocyanins of [[horseshoe crab]]s and some other species of [[arthropods]], cooperative binding is observed, with [[Hill coefficient]]s between 1.6 and 3. Hill constants vary depending on species and laboratory measurement settings. Hemoglobin, for comparison, has a Hill coefficient of usually 2.8 to 3. In these cases of [[cooperative binding]], hemocyanin was arranged in protein sub-complexes of 6 subunits (hexamer) each with one oxygen binding site; binding of oxygen on one unit in the complex would increase the affinity of the neighboring units. Each hexamer complex was arranged together to form a larger complex of dozens of hexamers. In one study, cooperative binding was found to be dependent on hexamers being arranged together in the larger complex, suggesting cooperative binding between hexamers. Hemocyanin oxygen-binding profile is also affected by dissolve-salt ion levels and [[pH]]. | ||
| + | |||
| + | Because of the large size of hemocyanin, it is usually found free-floating in the blood, unlike hemoglobin, which must be contained in cells because its small size would lead it to clog and damage blood-filtering organs such as the [[kidney]]s. This free-floating nature can allow for increased hemocyanin density over hemoglobin and increased oxygen carrying capacity. On the other hand, free-floating hemocyanin can increase viscosity and increase the energy expenditure needed to pump blood. | ||
==Immunotherapeutical effects== | ==Immunotherapeutical effects== | ||
| Line 34: | Line 36: | ||
==References== | ==References== | ||
| − | {{ | + | * Karlin, K. D., R. W. Cruse, Y. Gultneh, A. Farooq, J. C. Hayes and J. Zubieta | title = Dioxygen-copper reactivity. Reversible binding of O2 and CO to a phenoxo-bridged dicopper(I) complex | year = 1987 | journal = [[J. Am. Chem. Soc.]] | volume = 109 | issue = 9 | pages = 2668–2679 | doi=10.1021/ja00243a019}}</ref> |
| + | |||
| + | .<ref name = Kitajima>{{cite journal | author = N. Kitajima, K. Fujisawa, C. Fujimoto, Y. Morooka, S. Hashimoto, T. Kitagawa, K. Toriumi, K. Tatsumi and A. Nakamura | title = A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = isopropyl and Ph) | year = 1992 | journal = [[J. Am. Chem. Soc.]] | volume = 114 | issue = 4 | pages = 1277–1291 | doi=10.1021/ja00030a025}}</ref> | ||
Revision as of 23:39, 22 July 2008
Hemocyanin, or haemocyanin, is any of a group of copper-containing respiratory proteins that serve an oxygen-carrying function in the blood of some arthropods and most mollusks, similar to the role of hemoglobin found in the blood of vertebrates. Subunits of the hemocyanin chain contain two copper atoms that reversibly bind a single oxygen molecule (O2). Oxygenation causes a color change between the colorless Cu(I) deoxygenated form and the blue Cu(II) oxygenated form. Hemocyanins are second only to hemoglobin in biological popularity of use in oxygen transport.
Structure and mechanism
Hemocyanins use copper-binding sites to bind and transport oxygen. Hemocyanins typically have high molecular weights and are made of several individual subunit proteins, with each subunit containing two copper atoms and able to bind one oxygen molecule (O2). The subunits tend to aggregate. Oxygen affinity is affected by pH, temperature, and ionic concentration (Nigam et al. 1997).
The structure of arthropod hemocyanin tends to be quite different from that of mollusks (Nigam et al. 1997).
In arthropods, hemocyanin is composed of six subunits, or multiples of six subunits (Nigam et al. 1997). Such is the case, for example, in crayfish, lobsters, and crabs, where the structures are hexameric or dodecameric (protein complex with 12 protein subunits) (Nigam et al. 1997). Each subunit weighs about 75,000 daltons (75 kDa) and has two copper atoms. The subunits each have about three domains with oxygen bound in the second domain (Nigam et al. 1997). Each subunit of two copper atoms binds one molecule of O2.
In mollusks, the hemocyanin is about 290,000 daltons (290 kDa), with two copper units for each 50,000 daltons (Nigam et al. 1997). The polypeptide chain binds about six to eight O2 molecules (Nigam et al. 1997).
Spectroscopy of oxyhemocyanin shows several salient features:
- resonance Raman spectroscopy shows symmetric binding
- UV-Vis spectroscopy shows strong absorbencies at 350 and 580 nm.
- OxyHc is EPR-silent indicating the absence of unpaired electrons
- Infrared spectroscopy shows ν(O-O) of 755 cm-1
Feature one rules out a mononuclear peroxo complex. Feature two does not match with the UV-Vis spectra of mononuclear peroxo and Kenneth Karlin's trans-peroxo models (Karlin et al. 1987). Feature four shows a considerably weaker O-O bond compared with Karlin's trans-peroxo model (Karlin et al. 1987). On the other hand, Nobumasa Kitajima's model shows ν(O-O) of 741 cm-1 and UV-Vis absorbencies at 349 and 551 nm, which agree with the experimental observations for oxyHc (Kitajima et al. 1992). The weak O-O bond of oxyhemocyanin is because of metal-ligand backdonation into the σ* orbitals. The donation of electrons into the O-O antibonding orbitals weakens the O-O bond, giving a lower than expected infrared stretching frequency.
Hemocyanin versus hemoglobin
Although the respiratory function of hemocyanin is similar to that of hemoglobin, there are a significant number of differences in its molecular structure and mechanism. Whereas hemoglobin carries its iron atoms in porphyrin rings (heme groups), the copper atoms of hemocyanin are bound as prosthetic groups coordinated by histidine residues. Species using hemocyanin for oxygen transportation are commonly crustaceans living in cold environments with low oxygen pressure. Under these circumstances hemoglobin oxygen transportation is less efficient than hemocyanin oxygen transportation.
Most hemocyanins bind with oxygen non-cooperatively and are roughly one-fourth as efficient as hemoglobin at transporting oxygen per amount of blood. Hemoglobin binds oxygen cooperatively due to steric conformation changes in the protein complex, which increases hemoglobin's affinity for oxygen when partially oxygenated. In some hemocyanins of horseshoe crabs and some other species of arthropods, cooperative binding is observed, with Hill coefficients between 1.6 and 3. Hill constants vary depending on species and laboratory measurement settings. Hemoglobin, for comparison, has a Hill coefficient of usually 2.8 to 3. In these cases of cooperative binding, hemocyanin was arranged in protein sub-complexes of 6 subunits (hexamer) each with one oxygen binding site; binding of oxygen on one unit in the complex would increase the affinity of the neighboring units. Each hexamer complex was arranged together to form a larger complex of dozens of hexamers. In one study, cooperative binding was found to be dependent on hexamers being arranged together in the larger complex, suggesting cooperative binding between hexamers. Hemocyanin oxygen-binding profile is also affected by dissolve-salt ion levels and pH.
Because of the large size of hemocyanin, it is usually found free-floating in the blood, unlike hemoglobin, which must be contained in cells because its small size would lead it to clog and damage blood-filtering organs such as the kidneys. This free-floating nature can allow for increased hemocyanin density over hemoglobin and increased oxygen carrying capacity. On the other hand, free-floating hemocyanin can increase viscosity and increase the energy expenditure needed to pump blood.
Immunotherapeutical effects
The hemocyanin found in Concholepas concholepas blood has immunotherapeutic effects against bladder and prostate cancer. In a research made in 2006 mice were primed with C. concholepas before implantation of bladder tumor (MBT-2) cells. Mice treated with C. concholepas showed a significant antitumor effect as. The effects included prolonged survival, decreased tumor growth and incidence and lack of toxic effects.[1]
ReferencesISBN links support NWE through referral fees
- Karlin, K. D., R. W. Cruse, Y. Gultneh, A. Farooq, J. C. Hayes and J. Zubieta | title = Dioxygen-copper reactivity. Reversible binding of O2 and CO to a phenoxo-bridged dicopper(I) complex | year = 1987 | journal = J. Am. Chem. Soc. | volume = 109 | issue = 9 | pages = 2668–2679 | doi=10.1021/ja00243a019}}</ref>
.[2]
http://www.nyu.edu/projects/fitch/resources/student_papers/nigam.pdf
The Molecular Evolution of Arthropod & Molluscan Hemocyanin
Evidence for Apomorphic origin and convergent evolution in O2 hinding sites
Anupam N&am, Jimmy Ng and Trustin Ennacheril
December 1, 1997
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ [1] This Month in Investigative Urology, ScienceDirect
- ↑ N. Kitajima, K. Fujisawa, C. Fujimoto, Y. Morooka, S. Hashimoto, T. Kitagawa, K. Toriumi, K. Tatsumi and A. Nakamura (1992). A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = isopropyl and Ph). J. Am. Chem. Soc. 114 (4): 1277–1291.