Difference between revisions of "Goldenseal" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 68: | Line 68: | ||
===How goldenseal works=== | ===How goldenseal works=== | ||
| − | While most people assume that goldenseal has direct antimicrobial effects, it may work by more diffuse means. Herbalist [[Paul Bergner]] | + | While most people assume that goldenseal has direct antimicrobial effects, it may work by more diffuse means. Herbalist [[Paul Bergner]] reviewed the research and has been unable to find case reports where the level of intestinal pathogens are lower after taking goldenseal, although he has found many reports where symptoms were reduced (Bergner 1997a). In fact, a study by Rabbani et al. (1987), where men with ''[[E-coli]]'' induced diarrhea had 42-48% reduced symptoms after taking berberine, showed unchanged levels of intestinal bacteria, pathogenic or otherwise. Bergner concluded: |
| − | <blockquote>One traditional use of goldenseal is as a mucous membrane tonic. Note that it does not have to come in contact with the mucous membranes to have this effect. Hold some goldenseal in your mouth for a minute or two, and you can feel the effect on the mucous membranes in your nose and sinuses. Traditional doctors stated that goldenseal increases the secretion of the mucous membranes. At the same time, goldenseal contains astringent factors, which also counter that flow. Thus it was referred to as a mucous membrane "alterative" | + | <blockquote>One traditional use of goldenseal is as a mucous membrane tonic. Note that it does not have to come in contact with the mucous membranes to have this effect. Hold some goldenseal in your mouth for a minute or two, and you can feel the effect on the mucous membranes in your nose and sinuses. Traditional doctors stated that goldenseal increases the secretion of the mucous membranes. At the same time, goldenseal contains astringent factors, which also counter that flow. Thus it was referred to as a mucous membrane "alterative," increasing deficient flow but decreasing excessive flow. How this happens has not been determined by science, but is thoroughly supported by the traditional uses.... It is my opinion that goldenseal acts as an "antibiotic" to the mucous membranes not by killing germs directly, but by increasing the flow of healthy mucous, which contains its own innate antibiotic factors—[[IgA]] antibodies. This effect is unnecessary in the early stages of a cold or flu, when mucous is already flowing freely.</blockquote> |
| − | It appears likely that goldenseal shares with [[Mahonia]] (Oregon grape) and [[Berberis]] (Barberry) the ability to inhibit the drug resistance [[efflux pumps]] ([[MDR pumps]]) of bacteria | + | It appears likely that goldenseal shares with [[Mahonia]] (Oregon grape) and [[Berberis]] (Barberry) the ability to inhibit the drug resistance [[efflux pumps]] ([[MDR pumps]]) of bacteria. |
| + | ===Toxicity and cautions=== | ||
| + | Most of the research that is popularly attributed to goldenseal has actually been into the constituent [[berberine]], which goldenseal has in common with a variety of other medicines, including Oregon grape, coptis, phellodendron, and barberry. Constituents frequently act differently in isolation than a whole herb acts in the body. In 1996, the committee of the European Union that regulates drugs placed barberry (''[[Berberis vulgaris]]'') in a table of Herbal Drugs with Serious Risks without any Accepted Benefit because it contains berberine. This recommendation is at odds with the long traditional use of barberry and other berberine-containing herbs. Bergner (1997a), in a review of the literature, reported finding only a single report of potential adverse effects of berberis species, berberine-containing plants, or berberine itself in a computer search of the MEDLINE and TOXLINE databases of the U.S. National Library of Medicine. This was a study in China that showed that berberine sulfate is inappropriate for the treatment of newborn infants with prenatal jaundice (Chan 1993). However that is not a likely scenario in a country where babies born jaundiced are hospitalized, but it does lend credence to the traditional advice not to take goldenseal or other berberine herbs during pregnancy (Bergner 1997a). | ||
| − | + | Research into the toxicology and pharmacology of goldenseal has focused on berberine and hydrastine, which are antimicrobial, chloretic, and each have a variety of other properties helping immunity. But toxicity in a concentrated constituent does not translate to toxicity of the whole herb with its buffering compounds. In one study, the lethal dose (LD<sub>50</sub>) for rats was 12 times lower with hydrastine than with goldenseal extract (Tice 1997; Mills and Bone 2000). Authors of a study involving pregnant rats being fed about 47 times the usual human dose of 26 mg/kg concluded, "Maternal liver weights were increased at ≥6250 ppm, suggesting possible enzyme induction. There was no definitive evidence of developmental toxicity in this study" (NTP 2003). | |
| − | + | The lethal dose ([[LD50|LD<sub>50</sub>]]) of berberine isolates in humans is thought to be 27.5 mg/kg. Berberine is absorbed slowly orally; it achieves peak concentrations in 4 hours and takes 8 hours to clear (Jellin et al. 2004). Berberine is excreted in the urine, and human studies of berberine show evidence it can be absorbed through the skin. Berberine in humans can cause blocking of receptors in smooth muscle, blocking potassium channels in the heart and reducing [[ventricular tachycardia]], inhibiting intestinal ion secretion and toxin formation in the gut, and increasing [[bile]] secretion. | |
| − | |||
| − | |||
| − | + | While goldenseal, like all alkaloid-rich herbs including coffee and tobacco, should be avoided during pregnancy and given to very young children only with care, it appears that goldenseal is unlikely to be toxic in normal doses. Interactions with drugs with narrow therapeutic windows like [[Warfarin]], [[cyclosporin]], [[protease inhibitors]], and [[cardiac glycosides]] are potential concerns. | |
| − | + | According to Bergner (1997b), only 10% of goldenseal is used when it is appropriate and there are no better substitutes. Goldenseal has an affinity for mucosa, and is cooling so should not be used if an infection is at an early stage or there are more chills than fever. Goldenseal should be used with caution only while sick with illnesses that respond to hydrastine and berberine. It should generally not be taken for an early stage Upper Respiratory Infection (URI), but reserved for illnesses in which there is yellow or green phlegm. Generally a two week maximum dosage is suggested. Taking goldenseal over a long period of time can reduce absorption of B vitamins. Goldenseal should be avoided during pregnancy and lactation, as well as with gastrointestinal inflammation, and with proinflammatory disorders. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | proinflammatory disorders. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Endangered status== | ==Endangered status== | ||
| Line 110: | Line 94: | ||
==References== | ==References== | ||
| + | <ref name=autogenerated2>[http://www.medherb.com/84.HTM]Bergner, Paul ''Goldenseal and the Antibiotic Myth'' Medical Herbalism: A Journal for the Clinical Practitioner Volume 8, Number 4, Winter 1996-1997</ref> | ||
| + | <ref>Bergner, Paul.(1997b) ''The Healing Powers of Echinacea, Goldenseal and Other Immune System Herbs''. Prima ISBN-13: 978-0761508090</ref> | ||
| + | |||
| + | <ref>Chan, E. [http://www.ncbi.nlm.nih.gov/pubmed/8513024 Displacement of bilirubin from albumin by berberine] ''Biol Neonate'' 1993;63(4):201-8 PMID: 8513024</ref>. | ||
<ref>Foster S. and Duke J. (2000): ''A Field guide to Medicinal Plants and Herbs of Eastern and Central North America''.New York, Houghton Mifflin</ref> | <ref>Foster S. and Duke J. (2000): ''A Field guide to Medicinal Plants and Herbs of Eastern and Central North America''.New York, Houghton Mifflin</ref> | ||
| Line 118: | Line 106: | ||
* Hanrahan, C. 2005. Goldenseal. In J. Longe, ''The Gale Encyclopedia of Alternative Medicine,'' Farmington Hills, Mich: Thomson/Gale. ISBN 0787693960. | * Hanrahan, C. 2005. Goldenseal. In J. Longe, ''The Gale Encyclopedia of Alternative Medicine,'' Farmington Hills, Mich: Thomson/Gale. ISBN 0787693960. | ||
| + | |||
| + | <ref>Jellin J.M., Gregory P.J., Batz F. and Hitchens K. (2004): Pharmacist's Letter/ Prescriber's Letter Natural Medicines Comprehensive Database. Stockton, CA, Therapeutic Research Faculty. Accessed: 1/12/2004, http://www.naturaldatabase.com/member_home.asp?ph_img=memberhome.gif&ex=0&ex=0</ref> | ||
| Line 132: | Line 122: | ||
0443060169 | 0443060169 | ||
Mills and Bone | Mills and Bone | ||
| + | |||
| + | <ref name=NTP>(2003): [http://ntp-server.niehs.nih.gov/index.cfm?objectid=07312823-A6AC-E08E-0C0699ED7E97DCC2 Developmental Toxicity Evaluation for Goldenseal Root Powder (Hydrastis Canadensis) Administered in the Feed to Sprague-Dawley (CD) Rats on Gestational Days 6 to 20] Research Triangle Park, NC: National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health</ref> | ||
<ref>Tierra Michael (1998): The Way of Herbs. New York, Pocket Books</ref> <ref>Grieve M. (1971): A Modern Herbal. New York, Dover Publications, Inc</ref> <ref>Mills S. and Bone K. (2000): Principles and Practice of Phytotherapy. Philadelphia, Churchill Livingstone</ref> <ref name=autogenerated4>http://www.med.unc.edu/phyrehab/ncmedicinalherbs/goldenseal/Goldenseal-hp.pdf</ref> | <ref>Tierra Michael (1998): The Way of Herbs. New York, Pocket Books</ref> <ref>Grieve M. (1971): A Modern Herbal. New York, Dover Publications, Inc</ref> <ref>Mills S. and Bone K. (2000): Principles and Practice of Phytotherapy. Philadelphia, Churchill Livingstone</ref> <ref name=autogenerated4>http://www.med.unc.edu/phyrehab/ncmedicinalherbs/goldenseal/Goldenseal-hp.pdf</ref> | ||
| + | |||
| + | <ref>Rabbani, G.H., Butler, T., Knight, J., et al. ''Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli<D> and Vibrio cholerae''<D>. J Infectious Dis<D> 1987;155(5):979-984</ref> | ||
| + | |||
| + | <ref>Tice Raymond (1997): Goldenseal and Two of its constituent alkaloids: berberine and hydrastine Research Triangle Park, National Institute of Environmental Health Sciences, in Seiger E: ''Review of Toxilogical Literature''</ref><ref>Mills Simon and Bone Kerry (2000): '''Principles and Practice of Phytotherapy'''. Philadelphia, Churchill Livingstone</ref> | ||
Revision as of 20:42, 16 November 2008
| Goldenseal | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Hydrastis canadensis L. |
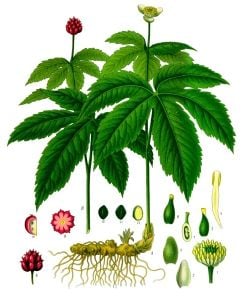
Goldenseal is the common name for a a perennial North American herb, Hydrastis canadensis, in the buttercup family Ranunculaceae, and characterized by a thick, yellow knotted rootstock, small greenish-white flowers, and two large lobed leaves with double-toothed edges. Also known as orange root (orange-root, orange root), this plant, which is native to southeastern Canada and eastern United States, is widely used in herbal medicine, mainly for chemicals from its root, but also the inner twig bark.
Goldenseal is considered a multiple-use remedy, being used for a wide variety of treatments.
Description
Hydrastis canadensis is a perennial herbaceous plant found wild in deciduous woods and damp meadows in eastern North America, from southeastern Canada, Vermont, and Minnesota in the north to Georgia and Arkansas in the south (Hanranah 2005). Each plant has only two large leaves, which are palmate and hairy, with 5 to 7 double-toothed lobes, serrated at the top edges. These two large leaves grow atop a forked, hairy stem. Each plant bears a single, small, inconspicuous flower with greenish white stamens in the late spring. Each goldenseal plant yields a single berry, like a large raspberry, with 10 to 30 seeds in the summer (Foster and Duke 2000).
The main medicinal part of goldenseal is the thick, yellow, knotted rootstock. It is commonly from 10 to 40 millimeters long and 3 to 12 millimeters thick (Kress 2008). The lower surface has many brittle, wiry roots, which are often broken off, leaving small, prominent scars, while the to bears numerous, short, upright branches (Kress 2008). The rhizome is hard; in traverse section it varies from dark yellow to dark yellowish-brown (Kress 2008). The stem is purplish and hairy above ground and yellow below ground where it connects to the yellow rhizome (horizontal stem). The hairy stem grows above ground to two feet (about 60 centimeters) in height.
Medicinal components
The rhizome, rootlets, and root hairs all produce medicinally active alkaloids (Tims and Batista 2007), as do the inner twig bark (Hanrahan 2005).
Goldenseal is rich in isoquinoline alkaloids, notably hydrastine, berberine, and canadine, as well as berberastine, hydrastinine, tetrahydroberberastine, and canalidine (Weber et al. 2003). A related compound, 8-oxotetrahydrothalifendine, was identified in one study (Gentry et al. 1998). Berberine and hydrastine act as quaternary bases and are poorly soluble in water but freely soluble in alcohol.
Hydrastine is found in proportion of about 1.5 to 3.2 percent, while berberine averages about 3 percent, and canadine is found only in a small quantity (Kress 2008). Other substances include resin, starch, and volatile oil.
Multiple bacteria and fungi, along with selected protozoa and chlamydia, are susceptible to berberine in vitro (Mills and Bone 2000). Berberine alone has weak antibiotic activity in vitro since many microorganisms actively export it from the cell. (Although a whole herb is likely to work on the immune system as well as on attacking the microbes and hence have a stronger clinical effect than the antibiotic activity alone would suggest). Interestingly, there is some evidence for other berberine-containing species synthesizing an efflux pump inhibitor that tends to prevent antibiotic resistance, a case of solid scientific evidence that the herb is superior to the isolated active principle (Lewis 2001). However, it is not yet known whether goldenseal contains a drug resistance efflux pump inhibitor, although many antimicrobial herbs do.
Medicinal uses
Goldenseal is used medicinally in many ways, both externally and internally, and has sometimes been called "poor man's ginseng" (Hanrahan 2005). Native American tribes traditionally valued this herb for medicinal uses and as a clothing dye, and early colonists appreciated its ability to fight infections (Hanrahan 2005). Today, it continues to be a popular herbal supplement for infections and pain.
Because of its variety of applications, goldenseal is considered a "cure-all medicinal herb" (Hanrahan 2005) or "multi-purpose remedy." A bitter herb, it is taken internally as a digestion aid, and for removal of canker sores when gargled. It is used as a topical antimicrobial and is considered effective against amoebic infection (Hanrahan 2005). It is used as anti-inflammatory, laxative, anticatarrhal, hepatic, emmenagogue, and oxytocic (Hoffman 2003). It is considered to stimulate the function of the liver, kidney, and lung, and is used in treatment of peptic ulcers (Hanrahan 2005). It is held to potentiate insulin and possibily help control bleeding. Externally applications have been used to treat gum disease, vaginal infection, impetigo, eczema, conjunctivitis, and inflammations of the ear (Hanrahan 2005). It may help lower blood pressure, and has been used as a remedy for diphtheria, typhoid fever, gonorrhea, syphilis, and tonsillitis (Hanrahan 2005).
Herbalists today consider goldenseal an alterative, anti-catarrhal, anti-inflammatory, antiseptic, astringent, bitter tonic, laxative, and muscular stimulant. They recommend goldenseal for gastritis, colitis, duodenal ulcers, loss of appetite, and liver disease. They discuss the astringent effect it has on mucous membranes of the upper respiratory tract, the gastrointestinal tract, the bladder, and rectum (applied topically), and the skin. Goldenseal is very bitter, which stimulates the appetite and aids digestion, and often stimulates bile secretion (Tierra 1998; Grieve 1971; Mills and Bone 2000).
Goldenseal may be purchased in salve, tablet, tincture form, or as a bulk powder. Goldenseal is often used to boost the medicinal effects of other herbs with which it is blended or formulated.
Traditional usage
At the time of the European conquest of the Americas, goldenseal was in extensive use among certain Native American tribes of North America, both as a medicine and as a coloring material. Benjamin Smith Barton, in his 1798 edition of Collections for an Essay Toward a Materia Medica of the United States, refers to the Cherokee use of goldenseal as a cure for cancer. Later, he calls attention to its properties as a bitter tonic, and as a local wash for ophthalmia. It became a favorite of the Eclectics from the time of Constantine Raffinesque in the 1830s.
Goldenseal was extensively used for cancers and swellings of the breasts by the Eclectics, although it was not considered sufficient for cancer alone. Hale recommended its use in hard swellings of the breast, while conium was used for smaller painless lumps. The two herbs alone or with phytoplankton Americana were used for cancers, along with alteratives like red clover.
Ellingwood's American Materia Medica lists goldenseal as being useful for functional disorders of the stomach, catarrhal gastritis, atonic dyspepsia, chronic constipation, hepatic congestion, cirrhosis, protracted fevers, cerebral engorgements of a chronic character, uterine subinvolution, in menorrhagia or metrorrhagia from the displaced uterus, post partum hemorrhage, catarrhal, ulcerating, aphthous, indolent and otherwise unhealthy conditions of mucous surfaces, leucorrhea, gallstones and breast swellings associated with the menses (Kress 2008).
Ellingwood cites one unusual use:
Cuthberton gave hydrastis canadensis as a tonic to a pregnant woman who had a goitre of recent appearance. The goitre was promptly cured. As a result of this observation, he treated twenty-five other cases of goitre at the time of puberty, or during the pregnant state. At times when interference with the function of the reproductive organs seemed to produce reflex irritation. He claims that every case was cured by this remedy. He gave the agent from six weeks to three months, three times a day after eating. One of the patients had become steadily worse under the use of iodine, the iodides, and thyroid extract. This patient began to improve as soon as hydrastis was given, and was promptly cured with this remedy alone.
How goldenseal works
While most people assume that goldenseal has direct antimicrobial effects, it may work by more diffuse means. Herbalist Paul Bergner reviewed the research and has been unable to find case reports where the level of intestinal pathogens are lower after taking goldenseal, although he has found many reports where symptoms were reduced (Bergner 1997a). In fact, a study by Rabbani et al. (1987), where men with E-coli induced diarrhea had 42-48% reduced symptoms after taking berberine, showed unchanged levels of intestinal bacteria, pathogenic or otherwise. Bergner concluded:
One traditional use of goldenseal is as a mucous membrane tonic. Note that it does not have to come in contact with the mucous membranes to have this effect. Hold some goldenseal in your mouth for a minute or two, and you can feel the effect on the mucous membranes in your nose and sinuses. Traditional doctors stated that goldenseal increases the secretion of the mucous membranes. At the same time, goldenseal contains astringent factors, which also counter that flow. Thus it was referred to as a mucous membrane "alterative," increasing deficient flow but decreasing excessive flow. How this happens has not been determined by science, but is thoroughly supported by the traditional uses.... It is my opinion that goldenseal acts as an "antibiotic" to the mucous membranes not by killing germs directly, but by increasing the flow of healthy mucous, which contains its own innate antibiotic factors—IgA antibodies. This effect is unnecessary in the early stages of a cold or flu, when mucous is already flowing freely.
It appears likely that goldenseal shares with Mahonia (Oregon grape) and Berberis (Barberry) the ability to inhibit the drug resistance efflux pumps (MDR pumps) of bacteria.
Toxicity and cautions
Most of the research that is popularly attributed to goldenseal has actually been into the constituent berberine, which goldenseal has in common with a variety of other medicines, including Oregon grape, coptis, phellodendron, and barberry. Constituents frequently act differently in isolation than a whole herb acts in the body. In 1996, the committee of the European Union that regulates drugs placed barberry (Berberis vulgaris) in a table of Herbal Drugs with Serious Risks without any Accepted Benefit because it contains berberine. This recommendation is at odds with the long traditional use of barberry and other berberine-containing herbs. Bergner (1997a), in a review of the literature, reported finding only a single report of potential adverse effects of berberis species, berberine-containing plants, or berberine itself in a computer search of the MEDLINE and TOXLINE databases of the U.S. National Library of Medicine. This was a study in China that showed that berberine sulfate is inappropriate for the treatment of newborn infants with prenatal jaundice (Chan 1993). However that is not a likely scenario in a country where babies born jaundiced are hospitalized, but it does lend credence to the traditional advice not to take goldenseal or other berberine herbs during pregnancy (Bergner 1997a).
Research into the toxicology and pharmacology of goldenseal has focused on berberine and hydrastine, which are antimicrobial, chloretic, and each have a variety of other properties helping immunity. But toxicity in a concentrated constituent does not translate to toxicity of the whole herb with its buffering compounds. In one study, the lethal dose (LD50) for rats was 12 times lower with hydrastine than with goldenseal extract (Tice 1997; Mills and Bone 2000). Authors of a study involving pregnant rats being fed about 47 times the usual human dose of 26 mg/kg concluded, "Maternal liver weights were increased at ≥6250 ppm, suggesting possible enzyme induction. There was no definitive evidence of developmental toxicity in this study" (NTP 2003).
The lethal dose (LD50) of berberine isolates in humans is thought to be 27.5 mg/kg. Berberine is absorbed slowly orally; it achieves peak concentrations in 4 hours and takes 8 hours to clear (Jellin et al. 2004). Berberine is excreted in the urine, and human studies of berberine show evidence it can be absorbed through the skin. Berberine in humans can cause blocking of receptors in smooth muscle, blocking potassium channels in the heart and reducing ventricular tachycardia, inhibiting intestinal ion secretion and toxin formation in the gut, and increasing bile secretion.
While goldenseal, like all alkaloid-rich herbs including coffee and tobacco, should be avoided during pregnancy and given to very young children only with care, it appears that goldenseal is unlikely to be toxic in normal doses. Interactions with drugs with narrow therapeutic windows like Warfarin, cyclosporin, protease inhibitors, and cardiac glycosides are potential concerns.
According to Bergner (1997b), only 10% of goldenseal is used when it is appropriate and there are no better substitutes. Goldenseal has an affinity for mucosa, and is cooling so should not be used if an infection is at an early stage or there are more chills than fever. Goldenseal should be used with caution only while sick with illnesses that respond to hydrastine and berberine. It should generally not be taken for an early stage Upper Respiratory Infection (URI), but reserved for illnesses in which there is yellow or green phlegm. Generally a two week maximum dosage is suggested. Taking goldenseal over a long period of time can reduce absorption of B vitamins. Goldenseal should be avoided during pregnancy and lactation, as well as with gastrointestinal inflammation, and with proinflammatory disorders.
Endangered status
Goldenseal is in serious danger due to overharvesting. Goldenseal became popular in the mid-nineteenth century. By 1905, the herb was much less plentiful, partially due to overharvesting and partially to habitat destruction. Wild goldenseal is now so rare that the herb is listed in the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES)[2] goldenseal is one of the most overharvested herbs. More than 60 million goldenseal plants are picked each year without being replaced.[3] The process of mountain top removal mining has recently put the wild goldenseal population at major risk due to loss of habitat, illegality of removing goldenseal for transplant without registration while destruction in the process of removing the mountain top is permitted, and increased economic pressure on stands outside of the removal area. [4]
There are several berberine-containing plants that can serve as useful alternatives, including Chinese coptis, yellowroot, or Oregon grape root.[5] Many herbalists urge caution in choosing products containing goldenseal, as they may have been harvested in an unsustainable manner as opposed to having been organically cultivated.
ReferencesISBN links support NWE through referral fees
[8].
- Hanrahan, C. 2005. Goldenseal. In J. Longe, The Gale Encyclopedia of Alternative Medicine, Farmington Hills, Mich: Thomson/Gale. ISBN 0787693960.
2008 Henriette Kress.
http://www.henriettesherbal.com/eclectic/bpc1911/hydrastis.html Hydrastis Rhizoma, B.P. Hydrastis Rhizome.
Henriette's Herbal Homepage
Hoffman David (2003): Medical Herbalism. Rochester, Vermont, Healing Arts Press</ref>
[13].
0443060169
Mills and Bone
http://www.springerlink.com/content/ug12932026810448/
Effects of Root Isoquinoline Alkaloids from Hydrastis canadensis on Fusarium oxysporum Isolated from Hydrastis Root Tissue
Journal Journal of Chemical Ecology Publisher Springer Netherlands ISSN 0098-0331 (Print) 1573-1561 (Online) Issue Volume 33, Number 7 / July, 2007 DOI 10.1007/s10886-007-9319-9 Pages 1449-1455 Michael C. Tims1, 3 Contact Information and Charisma Batista 2007
.[22]
Literature
- Blanchan, Neltje (2005). Wild Flowers Worth Knowing. Project Gutenberg Literary Archive Foundation.
- John Uri Lloyd (1908). Hydrastis canadensis. Lloyd Library, Cincinnati. PDF
- Bergner, Paul.(1997) The Healing Powers of Echinacea, Goldenseal and Other Immune System Herbs. Prima ISBN-13: 978-0761508090
- W. Scott Persons and Jeanine M. Davis (2005) Growing & Marketing Ginseng, Goldenseal & Other Woodland Medicinals Bright Mountain Books. ISBN-13: 978-0914875420
- Richo Cech. (2002) Growing At-Risk Medicinal Herbs, Cultivation, Conservation and Ecology ISBN-13: 978-0970031211
- University of North Carolina, Dept. of Integrative Medicine. Monograph on Goldenseal" http://www.med.unc.edu/phyrehab/ncmedicinalherbs/goldenseal/Goldenseal-hp.pdf
External links
- The Herbal Information Center
- About.com's Herb & Supplement Guide: Goldenseal
- Sustainable Source of Goldenseal
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ Hydrastis canadensis. NatureServe Explorer. NatureServe. Retrieved 2007-03-20.
- ↑ Foster Steven and Tyler Varro E. (1999): Tyler's Honest Herbal: A sensible guide to the use of herbs and related remedies. Binghamton, NY, The Haworth Herbal Press
- ↑ Where Have All the Flowers Gone? - herbal supplements threaten some herb species | Vegetarian Times | Find Articles at BNET.com
- ↑ http://www.herbalgram.org/herbalgram/articleview.asp?a=3080 Dean Myles Saving Wild Ginseng, Goldenseal, and other Native Plants from Mountain Top Removal. HerbalGram. 2007;73:50 © American Botanical Council
- ↑ Bergner, Paul. The Healing Powers of Echinacea, Goldenseal and Other Immune System Herbs. Prima 1997 ISBN 978-0761508090
- ↑ [1]Bergner, Paul Goldenseal and the Antibiotic Myth Medical Herbalism: A Journal for the Clinical Practitioner Volume 8, Number 4, Winter 1996-1997
- ↑ Bergner, Paul.(1997b) The Healing Powers of Echinacea, Goldenseal and Other Immune System Herbs. Prima ISBN-13: 978-0761508090
- ↑ Chan, E. Displacement of bilirubin from albumin by berberine Biol Neonate 1993;63(4):201-8 PMID: 8513024
- ↑ Foster S. and Duke J. (2000): A Field guide to Medicinal Plants and Herbs of Eastern and Central North America.New York, Houghton Mifflin
- ↑ Gentry E. J., Jampani H. B., Keshavarz-Shokri A., Morton M. D., Velde D. V., Telikepalli H., Mitscher L. A., Shawar R., Humble D. and Baker W. (1998): Antitubercular natural products: berberine from the roots of commercial Hydrastis canadensis powder. Isolation of inactive 8-oxotetrahydrothalifendine, canadine, beta-hydrastine, and two new quinic acid esters, hycandinic acid esters-1 and -2. J Nat Prod. 61(10): 1187-93
- ↑ Jellin J.M., Gregory P.J., Batz F. and Hitchens K. (2004): Pharmacist's Letter/ Prescriber's Letter Natural Medicines Comprehensive Database. Stockton, CA, Therapeutic Research Faculty. Accessed: 1/12/2004, http://www.naturaldatabase.com/member_home.asp?ph_img=memberhome.gif&ex=0&ex=0
- ↑ Lewis K. (2001): In search of natural substrates and inhibitors of MDR pumps. J Mol Microbiol Biotechnol. 3(2): 247-54.
- ↑ Mills Simon and Bone Kerry (2000): Principles and Practice of Phytotherapy. Philadelphia, Churchill Livingstone
- ↑ (2003): Developmental Toxicity Evaluation for Goldenseal Root Powder (Hydrastis Canadensis) Administered in the Feed to Sprague-Dawley (CD) Rats on Gestational Days 6 to 20 Research Triangle Park, NC: National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health
- ↑ Tierra Michael (1998): The Way of Herbs. New York, Pocket Books
- ↑ Grieve M. (1971): A Modern Herbal. New York, Dover Publications, Inc
- ↑ Mills S. and Bone K. (2000): Principles and Practice of Phytotherapy. Philadelphia, Churchill Livingstone
- ↑ http://www.med.unc.edu/phyrehab/ncmedicinalherbs/goldenseal/Goldenseal-hp.pdf
- ↑ Rabbani, G.H., Butler, T., Knight, J., et al. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli<D> and Vibrio cholerae<D>. J Infectious Dis<D> 1987;155(5):979-984
- ↑ Tice Raymond (1997): Goldenseal and Two of its constituent alkaloids: berberine and hydrastine Research Triangle Park, National Institute of Environmental Health Sciences, in Seiger E: Review of Toxilogical Literature
- ↑ Mills Simon and Bone Kerry (2000): Principles and Practice of Phytotherapy. Philadelphia, Churchill Livingstone
- ↑ Weber H. A., Zart M. K., Hodges A. E., Molloy H. M., O'Brien B. M., Moody L. A., Clark A. P., Harris R. K., Overstreet J. D. and Smith C. S. (2003): Chemical comparison of goldenseal (Hydrastis canadensis L.) root powder from three commercial suppliers. J Agric Food Chem. 51(25): 7352-8.
