Atropine
| |
| |
Atropine
| |
| Systematic name | |
| IUPAC name (8-methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate | |
| Identifiers | |
| CAS number | 51-55-8 |
| ATC code | A03BA01 S01FA01 |
| PubChem | 174174 |
| DrugBank | APRD00807 |
| Chemical data | |
| Formula | C17H23NO3 |
| Mol. weight | 289.369 |
| Pharmacokinetic data | |
| Bioavailability | 25% |
| Metabolism | 50% hydrolysed to tropine and tropic acid |
| Half life | 2 hours |
| Excretion | 50% excreted unchanged in urine |
| Therapeutic considerations | |
| Pregnancy cat. | ? |
| Legal status | Rx only |
| Routes | Oral, IV, rectal |
Atropine is an alkaloid (naturally occurring amine produced by a plant) extracted from the deadly nightshade (Atropa belladonna) and other plants of the nightshade family (Solanaceae). It is a secondary metabolite of these plants and serves as a drug with a wide variety of effects. As it is potentially deadly, it derives its name from Atropos, one of the three Fates who, according to Greek mythology, chose how a person was to die.
Human creativity has developed the ability to commercial prepare and utilize atropine for a variety of medical purposes. These include keeping air passages clear and preventing heart slowing during anesthesia; dilating pupils of the eye for ophthalmology; providing symptomatic relief of colds and asthma; treating bradycardia (extremely slow heart rate) and heart block; serving as an antidote to certain poisons, such as nerve gas; and acting as an antisposmadic. However, human creativity also can be used for ill effect, as seen in the fact that atropine, because of its sometimes hallucinogenic properties, has been used as a rather dangerous recreational drug.
Description
Atropine is an alkaloid with the chemical formula C17H23NO3. It belongs to the tropane group of alkaloids, with tropane being a nitrogenous bicyclic organic compound with chemical formula C8H15N. Tropine is mainly known for a group of alkaloids derived from it (called tropane alkaloids), which include, among others, atropine, cocaine, and scopolamine (also known as hyoscine).
Atropine is obtained from such solanaceous plants as Atropa belladonaa (deadly nightshade), Hyoscyamus niger (black henbane), and Datura stramonium (thornapple) (Blakemore and Jennett 2001). These plants contain two closely related alkaloids, hyoscyamine and hyoscine, and atropine is a mixture of two isomers of hyoscyamine (Blakemore and Jennett 2001). That is, atropine is a racemic mixture of the alkaloids D-hyoscyamine and L-hyoscyamine, with most of its physiological effects due to L-hyoscyamine. Commercially, it is manufactured largely by using L-hyoscyamine, taken from Hyoscyamus niger, and partially converting this enantiomer into the D form (D-hyoscyamine). It is sometimes known as dl-hyoscyamine. Other plants of the Solanaceae family that naturally contain minute amounts of atropine include Atropa betica, Datura innoxia, Datura niger, and members of the Brugmansia genus. The Nicotiana genus (including the tobacco plant, N. tabacum) is also found in the Solanaceae family, but these plants do not contain atropine or other tropane alkaloids.
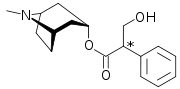
Atropine's systematic chemical name is 8-methyl-8-azabicyclo[3.2.1]oct-3-yl) 3-hydroxy-2-phenylpropanoate.
Its pharmacological effects are due to its binding to muscarinic acetylcholine receptors. It is an antimuscarinic agent.
The most common atropine compound used in medicine is atropine sulfate (C17H23NO3)2·H2SO4·H2O, the full chemical name is 1α H, 5α H-Tropan-3-α ol (±)-tropate(ester), sulfate monohydrate.
History
Mandragora (mandrake) of the nightshade family (Solanaceae) was described by Theophrastus in the fourth century B.C.E. for treatment of wounds, gout, and sleeplessness, and as a love potion. By the first century C.E., Dioscorides recognized wine of mandrake as an anesthetic for treatment of pain or sleeplessness, to be given prior to surgery or cautery (Holzman 1998). The use of Solanaceae containing tropane alkaloids for anaesthesia, often in combination with opium, persisted throughout the Roman and Islamic Empires and continued in Europe until superseded by the use of ether, chloroform, and other modern anesthetics.
Atropine extracts from the Egyptian henbane were used by Cleopatra in the last century B.C.E. to dilate her pupils, in the hope that she would appear more alluring. In the Renaissance, women used the juice of the berries of Atropa belladonna to enlarge the pupils of their eyes, for cosmetic reasons; bella donna is Italian for "beautiful lady." It likewise is said that Spanish ladies put atropine drops in their eyes to create the allure of large, black pupils (Blakemore and Jennett 2001).
The mydriatic effects of atropine were studied, among others, by the German chemist Friedrich Ferdinand Runge (1795–1867). In 1831, the pharmacist Mein succeeded in developing the pure crystalline isolation of atropine. The substance was first synthesized by German chemist Richard Willstätter in 1901.
Physiological effects and uses
Generally, atropine lowers the "rest and digest" activity of all muscles and glands regulated by the parasympathetic nervous system, including the heart, glandular tissue, and smooth muscle. This occurs because atropine is a competitive antagonist of the muscarinic acetylcholine receptors; that is, atropine blocks the action of acetylcholine at all nerve endings where the membrane receptors are of the muscarinic type (Blakemore and Jennett 2001). Acetylcholine is the main neurotransmitter used by the parasympathetic nervous system. Therefore, atropine may cause swallowing difficulties and reduced secretions (such as saliva and digestive enzymes), a rise in heart rate, and a relaxing of the smooth muscle of the gastrointestinal tract, the urinary bladder, and the bronchial trees (Blakemore and Jennett 2001). The central nervous system also contains muscarinic receptors and blockage of these by atropine can lead to restlessness and mental excitement, and large doses can cause hallucination.
Ophthalmic use
Topical atropine is used as a cycloplegic, to temporarily paralyze the accommodation reflex, and as a mydriatic, to dilate the pupils with long-lasting effect. Atropine degrades slowly, typically wearing off in 2 to 3 days, so tropicamide and phenylephrine are generally preferred as mydriatics. The effects of atropine can last up to two weeks.
The iris has both circular and radial muscles that work in a complementary manner to control the pupil diameter. In atropine-induced mydriasis, the mechanism of action involves blocking the contraction of the circular pupillary sphincter muscle, which is normally stimulated by acetylcholine release, thereby allowing the radial pupillary dilator muscle to contract and dilate the pupil. Atropine is contraindicated in patients predisposed to narrow angle glaucoma.
Atropine can be given to patients who have direct globe trauma.
Resuscitation
Injections of atropine are used in the treatment of bradycardia (extremely low heart rate), asystole, and pulseless electrical activity (PEA) in cardiac arrest. This works because the main action of the vagus nerve of the parasympathetic system on the heart is to slow it down. Atropine blocks that action and therefore may speed up the heart rate. The usual dose of atropine is 0.5 to 1 mg every three to five minutes, up to a maximum dose of 3 mg.
Atropine is also useful in treating first degree heart block, second degree heart block Mobitz Type 1 (Wenckebach block), and also third degree heart block with a high Purkinje or AV-nodal escape rhythm. It is usually not effective in second degree heart block Mobitz type 2, and in third degree heart block with a low Purkinje or ventricular escape rhythm. Atropine is contraindicated in ischemia-induced conduction block, because the drug increases oxygen demand of the AV nodal tissue, thereby aggravating ischemia and the resulting heart block.
One of the main actions of the parasympathetic nervous system is to stimulate the M2 muscarinic receptor in the heart, but atropine inhibits this action.
Secretions and bronchoconstriction
Atropine's actions on the parasympathetic nervous system inhibits salivary, sweat, and mucus glands. This can be useful in treating Hyperhidrosis and can prevent the death rattle of dying patients. Even though it has not been officially indicated for either of these purposes by the FDA, it has been used by physicians for these purposes (Bickel et al. 2019).
Antidote for organophosphate poisoning
By blocking the action of acetylcholine at muscarinic receptors, atropine also serves as an antidote for poisoning by organophosphate insecticides and nerve gases. Troops who are likely to be attacked with chemical weapons often carry autoinjectors with atropine and obidoxime, which can be quickly injected into the thigh. It is often used in conjunction with Pralidoxime chloride.
Atropine is given as an antidote to SLUDGE (Salivation, Lacrimation, Urination, Diaphoresis, Gastrointestinal motility, Emesis) symptoms caused by organophosphate poisoning.
Some of the nerve gases attack and destroy acetylcholinesterase, so the action of acetylcholine becomes prolonged. Therefore, atropine can be used to reduce the effect of acetylcholine.
Side effects and overdose
Adverse reactions to atropine include ventricular fibrillation, supraventricular or ventricular tachycardia, dizziness, nausea, blurred vision, loss of balance, dilated pupils, photophobia, and possibly, notably in the elderly, extreme confusion, hallucinations, and excitation. These latter effects are due to the fact that atropine is able to cross the blood-brain barrier. Because of the hallucinogenic properties, some have used the drug recreationally, though this is very dangerous and often unpleasant.
In overdoses, atropine is poisonous. Atropine is sometimes added to other potentially addictive drugs; abuse of those drugs is then prevented by the unpleasant effects of atropine overdose.
The antidote to atropine is physostigmine or pilocarpine.
A commonly used mnemonic used to described the physiologic manifestations of atropine overdose is: "hot as a hare, blind as a bat, dry as a bone, red as a beet, and mad as a wet hen" (Holzman 1998). This set of symptoms is known as anticholinergic toxidrome, and may also be caused by other drugs with anticholinergic effects, such as diphenhydramine, phenothiazine antipsychotics, and benztropine (Broderick, Metheny, Crosby 2023).
ReferencesISBN links support NWE through referral fees
- Blakemore, C. and S. Jennett. 2001. The Oxford Companion to the Body. New York: Oxford University Press. ISBN 019852403X
- Broderick, E.D., H. Metheny, and B. Crosby. 2023. Anticholinergic Toxicity. National Library of Medicine. Retrieved April 2, 2025.
- Bickel, K., L. Kareem, T. Bui, and R.M. Arnold. 2019. FF #109 Death Rattle and Oral Secretions. Palliative Care Network of Wisconsin. Retrieved April 2, 2025.
- Holzman, R.S. 1998. The legacy of Atropos, the fate who cut the thread of life. Anesthesiology 89(1): 241-249. Retrieved April 2, 2025.
External links
All links retrieved April 2, 2025.
- Atropine injection Cleveland Clinic
- Atropine Ophthalmic Medline Plus
- Atropine RxList
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.