Nitrate
- Trinitrate redirects here. See also glyceryl trinitrate.
In inorganic chemistry, a nitrate is a salt of nitric acid with an ion comprised of one nitrogen and three oxygen atoms. In organic chemistry the esters of nitric acid and various alcohols are called nitrates.
Chemical properties
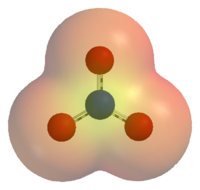
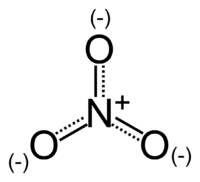
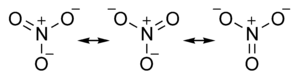
The nitrate ion is a polyatomic ion with the empirical formula NO3− and a molecular mass of 62.0049. It consists of one central nitrogen atom surrounded by three identical oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a negative one formal charge and can be represented as a hybrid of the following resonance structures:
The nitrate ion is the conjugate base of nitric acid. A nitrate salt forms when a positively charged ion attaches to the negatively charged oxygen atoms of the ion, forming an ionic compound. Almost all nitrates are soluble in water at standard temperature and pressure.
In organic chemistry a nitrate is a functional group with general chemical formula RONO2 where R stands for any organic residue. They are the esters of nitric acid and alcohols formed by nitroxylation. Examples are methyl nitrate formed by reaction of methanol and nitric acid [1], the nitrate of tartaric acid [2] and the inappropriately named nitroglycerin.
Occurrence and history

Solid nitrates are not very abundant in Nature as they are very soluble. They can appear where nitrogen-containing ground water is evaporating (e.g. in soils of arid regions, on animal shed walls). Nitrification bacteria in the soil are also needed for the process. The first commercially exploited source was India. While the British Empire had a reliable supply, the continental powers had to collect scrapings from walls and barns, install saltpetre farms (based on aging and leaching manure and urine). The famed tax-collecting duties of Lavoisier actually consisted of being the commissioner of this Saltpeter Administration. Later the large deposits of sodium nitrate in the Atacama Desert of northern Chile acquired economic significance.
Until early in the 20th century there was no means of chemical synthesis of nitrates. Chile was a major exporter, and European countries with burgeoning populations due to the industrial revolution were dependent on its nitrates for use as fertilizer to feed their people. They were needed for modern military explosives as well. These two critical uses proved to be crucial in world history — almost. Had the Germans not devised the Haber and Ostwald processes for producing nitrate, they would not have been able to feed their civilian population and armies, nor continued to make explosives. The First World War might have ended as a direct result of embargo of essential raw materials. With the aid of organic chemistry, however, the war continued. Nowadays most nitrates are produced from ammonia synthesized from atmospheric nitrogen.
Related materials
Nitrates should not be confused with nitrites, the salts of nitrous acid. Organic compounds containing the nitro functional group (which has the same formula and structure as the nitrate ion save that one of the O− atoms is replaced by the R group) are known as nitro compounds.
Effects on aquatic life
In freshwater or estuarine systems close to land, nitrate can reach high levels that can potentially cause the death of fish. While nitrate is much less toxic than ammonia or nitrite, levels over 30 ppm of nitrate can inhibit growth, impair the immune system and cause stress in some aquatic species[citation needed]. In most cases of excess nitrate concentrations, the principal pathway of entering aquatic systems is through surface runoff from agricultural or landscaped areas which have received excess nitrate fertilizer. These levels of nitrate can also lead to algae blooms, and when nutrients become limiting (such as potassium, phosphate or nitrate) then eutrophication can occur. As well as leading to water anoxia, these blooms may cause other changes to ecosystem function, favouring some groups of organisms over others. Consequently, as nitrates form a component of total dissolved solids, they are widely used as an indicator of water quality.
See also
- F-ratio
- Nitrification
External links
ReferencesISBN links support NWE through referral fees
cs:Dusičnany da:Nitrat de:Nitrate es:Nitrato fr:Nitrate gl:Nitrato it:Nitrati nl:Nitraat nn:Nitrat ug:ئازوتلۇق تۇز pl:Azotany pt:Nitrato ru:Селитры sr:Нитрат fi:Nitraatti sv:Nitrat