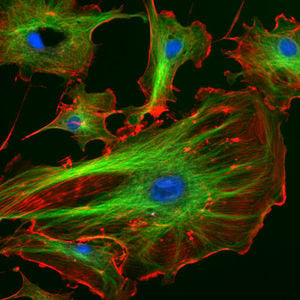
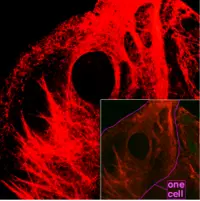
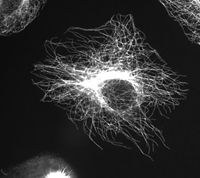
Cytoskeleton
The cytoskeleton (CSK) is a complex, three-dimensional network of protein filaments that extends throughout the cytoplasm of cells acting as a cellular "scaffolding" or "skeleton." This internal framework of protein filaments is a dynamic structure that gives cells their various shapes, provides a basis for coordinated and directed movement of cells (using structures such as flagella, cilia and lamellipodia), plays an important role in intracellular movement and integration of organelles and other sub-cellular structures in the cytoplasm, often protects the cell, and is involved in cell division and chromosome organization and movement (Alberts et al. 1989).
There are three main types of cytoskeletal filaments: actin filaments, microtubules, and intermediate filaments. In animal cells, the cytoskeleton often is organized from a region near the nucleus where is located the cell's pair of centrioles (Alberts et al. 1989).
The cytoskeleton once was once thought to be unique to eukaryotic cells, but recent research has identified cytoskeletal structures in bacteria, with homologs to all three of the major types of cytoskeletal proteins (actin, tubulin, and intermediate fiber proteins) (Shih and Rothfield 2006).
The eukaryotic cytoskeleton
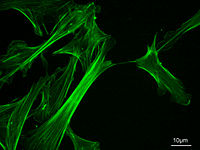
Eukaryotic cells contain three main kinds of cytoskeletal filaments, which are microfilaments or actin filaments, intermediate filaments, and microtubules. These filaments networked together provide the cell's cytoplasm with structure and shape.
Actin filaments / Microfilaments
Commonly around 8 nanometers (nm) in diameter, this filament is composed of two intertwined actin chains. Actin is a globular structural protein. It polymerizes in a helical fashion to form an actin filament (or microfilament). Actin genes are similar in different organisms and thus actin molecules from different sources are functionally interchangeable in laboratory tests (Alberts et al. 1989).
Actin filaments are most concentrated just beneath the cell membrane. They appear in electron microscopy as uniform threads about 8 nm wide (Alberts et al. 1989). Composed of a tight helix of uniformly oriented actin monomers, the actin filament is a polar structure, with two structurally different ends (Alberts et al. 1989).
Microfilaments are responsible for resisting tension, providing mechanical support for the cell, and determining cellular shape; enabling cell movements through forming cytoplasmatic protuberances (like pseudopodia and microvilli—although these by different mechanisms); and participation in some cell-to-cell or cell-to-matrix junctions. In association with these latter roles, microfilaments are essential to transduction. They are also important for cytokinesis (specifically, formation of the cleavage furrow) and, along with myosin, muscular contraction. Actin/myosin interactions also help produce cytoplasmic streaming in most cells.
Intermediate filaments
Intermediate filaments (IF), 8 to 12 nanometers in diameter, are more stable (strongly bound) than actin filaments, and heterogeneous constituents of the cytoskeleton. They are formed of four types of fibrous polypeptides. Type I IF proteins include two subfamilies of keratins, acidic keratins and neutral or basic keratins (Alberts et al. 1989). These are found primarily in epithelial cells (skin cells, hair and nails). Type II IF proteins include vimentin, desmin, and glial fibrillary acidic protein, and are the common structure support of many cells, including respectively, cells of mesenchymal origin, muscle cells, and glial cells (Alberts et al. 1989). Type III IF proteins are neurofilament proteins, and are a major cytoskeletal component in neurons (nerve axons and dendrites) (Alberts et al. 1989). Type IV IF proteins are the nuclear lamins, which form highly organized, two-dimensional sheets of filaments and are part of the nuclear lamina of cells (Alberts et al. 1989). All eukaryotic cells make nuclear lamins and usually at least one additioanl type of IF protein (Alberts et al. 1989).
Like actin filaments, intermediate filaments function in the maintenance of cell-shape by bearing tension. (Microtubules, by contrast, resist compression. It may be useful to think of micro- and intermediate filaments as cables, and of microtubules as cellular support beams.) Intermediate filaments organize the internal tridimensional structure of the cell, anchoring organelles and serving as structural components of the nuclear lamina and sarcomeres. They also participate in some cell-cell and cell-matrix junctions.
Microtubules
Microtubules are hollow cylinders about 25 nm in diameter (lumen = approximately 15nm in diameter), most commonly comprised of 13 protofilaments which, in turn, are polymers of alpha and beta tubulin.
More specifically, tubulin is a heterodimer of alpha and beta tubulin (both composed of about 450 amino acids). The tubulin molecules form linear protofilaments with the beta tubulin subunit of one tubulin molecule in contact with the alpha tubulin subunit of the next. The 13 protofilaments are arranged side by side around a central core that appears to be hollow, with the alignment in parallel, with the same polarity, resulting in the microtubule being a polar structure with a plus and minus end (Alberts et al. 1989).
Microtubules have a very dynamic behavior, binding GTP for polymerization. They are commonly organized by the centrosome.
In nine triplet sets (star-shaped), they form the centrioles, and in nine doublets oriented about two additional microtubules (wheel-shaped) they form cilia and flagella. The latter formation is commonly referred to as a "9+2" arrangement, wherein each doublet is connected to another by the protein dynein. As both flagella and cilia are structural components of the cell, and are maintained by microtubules, they can be considered part of the cytoskeleton.
Microtubules play key roles in intracellular transport (associated with dyneins and kinesins, they transport organelles like mitochondria or vesicles); the axoneme of cilia and flagella; the mitotic spindle; and synthesis of the cell wall in plants.
Comparison
| Cytoskeleton type | Diameter (nm) (Walter 2003) |
Structure | Subunit examples (Walter 2003) |
|---|---|---|---|
| Microfilaments | 8-10 | double helix | actin |
| Intermediate filaments | 8-10 | two parallel helices/dimers, forming tetramers |
|
| Microtubules | 25 | protofilaments, in turn consisting of tubulin subunits | α- and β-tubulin |
Microtrabeculae - a further structural network?
A fourth eukaryotic cytoskeletal element, microtrabeculae, was proposed by Keith Porter based on images obtained from high-voltage electron microscopy of whole cells in the 1970s. The images showed short, filamentous structures of unknown molecular composition associated with known cytoplasmic structures. Porter proposed that this microtrabecular structure represented a novel filamentous network distinct from microtubules, filamentous actin, or intermediate filaments. It is now generally accepted that microtrabeculae are nothing more that an artefact of certain types of fixation treatment though we have yet to fully understand the complexity of the cell's cytoskeleton[1].
The prokaryotic cytoskeleton
The cytoskeleton was previously thought to be a feature only of eukaryotic cells, but homologues to all the major proteins of the eukaryotic cytoskeleton have recently been found in prokaryotes.[2] Although the evolutionary relationships are so distant that they are not obvious from protein sequence comparisons alone, the similarity of their three-dimensional structures and similar functions in maintaining cell shape and polarity provides strong evidence that the eukaryotic and prokaryotic cytoskeletons are truly homologous.[3]
- "In recent years it has been shown that bacteria contain a number of cytoskeletal structures. The bacterial cytoplasmic elements include homologs of the three major types of eukaryotic cytoskeletal proteins (actin, tubulin, and intermediate filament proteins) and a fourth group, the MinD-ParA group, that appears to be unique to bacteria. The cytoskeletal structures play important roles in cell division, cell polarity, cell shape regulation, plasmid partition, and other functions." Shuh and Rothfield 2006
FtsZ
FtsZ was the first protein of the prokaryotic cytoskeleton to be identified. Like tubulin, FtsZ forms filaments in the presence of GTP, but these filaments do not group into tubules. During cell division, FtsZ is the first protein to move to the division site, and is essential for recruiting other proteins that synthesize the new cell wall between the dividing cells.
MreB and ParM
Prokaryotic actin-like proteins, such as MreB, are involved in the maintenance of cell shape. All non-spherical bacteria have genes encoding actin-like proteins, and these proteins form a helical network beneath the cell membrane that guides the proteins involved in cell wall biosynthesis.
Some plasmids encode a partitioning system that involves an actin-like protein ParM. Filaments of ParM exhibit dynamic instability, and may partition plasmid DNA into the dividing daughter cells by a mechanism analogous to that used by microtubules during eukaryotic mitosis.
Crescentin
The bacterium Caulobacter crescentus contains a third protein, crescentin, that is related to the intermediate filaments of eukaryotic cells. Crescentin is also involved in maintaining cell shape, but the mechanism by which it does this is currently unclear.
ReferencesISBN links support NWE through referral fees
- ↑ Heuser J (2002). Whatever happened to the 'microtrabecular concept'?. Biol Cell 94 (9): 561–96.
- ↑ Shih YL, Rothfield L (2006). The bacterial cytoskeleton. Microbiol. Mol. Biol. Rev. 70 (3): 729–54.
- ↑ Michie KA, Löwe J (2006). Dynamic filaments of the bacterial cytoskeleton. Annu. Rev. Biochem. 75: 467–92.
- Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. Molecular Biology of the Cell, 2nd edition. New York: Garland Publishing, 1989. ISBN 0824036956.
homologues to all the major proteins of the eukaryotic cytoskeleton have recently been found in prokaryotes.[1]
Further reading
- Linda A. Amos and W. Gradshaw Amos, Molecules of the Cytoskeletion, Guilford, ISBN 0-89862-404-5, LoC QP552.C96A46 1991
External links
- Cytoskeleton, Cell Motility and Motors - The Virtual Library of Biochemistry and Cell Biology
- Cytoskeleton database, clinical trials, recent literature, lab registry ...
- Animation of leukocyte adhesion (Animation with some images of actin and microtubule assembly and dynamics.)
| Proteins of the Cytoskeleton |
|---|
| Microfilaments - Actins | Myosins | Actin-binding proteins |
| Prokaryotic cytoskeleton - FtsZ | MreB | Crescentin |
| Intermediate filaments - Keratins | Type III IF proteins | Neurofilaments | Lamins | Intermediate filament-associated proteins |
| Microtubules - Tubulins | Dyneins | Kinesins | Microtubule-associated proteins |
| Other - Major Sperm Proteins |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ Shih YL, Rothfield L (2006). The bacterial cytoskeleton. Microbiol. Mol. Biol. Rev. 70 (3): 729–54.
- ↑ Unless else specified in boxes, then ref is:Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders, 1300. ISBN 1-4160-2328-3. Page 25