Difference between revisions of "Cortisone" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 5: | Line 5: | ||
| align="center" colspan="2" bgcolor="#ffffff" | [[Image:Cortisone-2D-skeletal.png|200px|Chemical structure of cortisone]] | | align="center" colspan="2" bgcolor="#ffffff" | [[Image:Cortisone-2D-skeletal.png|200px|Chemical structure of cortisone]] | ||
|- | |- | ||
| − | | [[IUPAC nomenclature|Systematic name]] | + | | [[IUPAC nomenclature|Systematic name]]* |
| 17,21-dihydroxypregn-4-ene-3,11,20-trione | | 17,21-dihydroxypregn-4-ene-3,11,20-trione | ||
|- | |- | ||
| − | | [[Chemical formula]] | + | | [[Chemical formula]]* |
| C<sub>21</sub>H<sub>28</sub>O<sub>5</sub> | | C<sub>21</sub>H<sub>28</sub>O<sub>5</sub> | ||
|- | |- | ||
| − | | [[Molecular mass]] | + | | [[Molecular mass]]* |
| 360.46 g/mol | | 360.46 g/mol | ||
|- | |- | ||
| − | | [[Density]] | + | | [[Density]]* |
| ? g/cm<sup>3</sup> | | ? g/cm<sup>3</sup> | ||
|- | |- | ||
| − | | [[Melting point]] | + | | [[Melting point]]* |
| 220-224 °C | | 220-224 °C | ||
|- | |- | ||
| − | | [[CAS registry number|CAS number]] | + | | [[CAS registry number|CAS number]]* |
| [53-06-5] | | [53-06-5] | ||
|- | |- | ||
| − | | [[Simplified molecular input line entry specification|SMILES]] | + | | [[Simplified molecular input line entry specification|SMILES]]* |
| <small>C[C@@](C3)4[C@](CC[C@@](O)4<br>[C@@](CO)=O)([H])[C@]2([H])CCC1=CC<br>(CC[C@@](C)1[C@]([H])2C3=O)=O</small> | | <small>C[C@@](C3)4[C@](CC[C@@](O)4<br>[C@@](CO)=O)([H])[C@]2([H])CCC1=CC<br>(CC[C@@](C)1[C@]([H])2C3=O)=O</small> | ||
|- | |- | ||
| Line 30: | Line 30: | ||
|} | |} | ||
| − | '''Cortisone''' (17-hydroxy-11-dehydrocorticosterone) is a naturally occuring [[steroid]] [[hormone]] that functions in carbohydrate metabolism and is used medically for the treatment of various ailments, including rheumatoid arthritis and certain allergies. Its formula is formula C<sub>21</sub>H<sub>28</sub>O<sub>5</sub> and IUPAC name is 17,21-dihydroxypregn-4-ene-3,11,20-trione. | + | '''Cortisone''' (17-hydroxy-11-dehydrocorticosterone) is a naturally occuring [[steroid]] [[hormone]] that functions in [[carbohydrate]] [[metabolism]] and is used medically for the treatment of various ailments, including rheumatoid [[arthritis]] and certain [[allergy|allergies]]. Its formula is formula C<sub>21</sub>H<sub>28</sub>O<sub>5</sub> and IUPAC name is 17,21-dihydroxypregn-4-ene-3,11,20-trione. |
| − | Cortisone is a | + | Cortisone is a corticosteroid, a term that refers to steroid hormones that are produced in the [[adrenal gland|adrenal cortex]] of the body. Cortisone is essential for life. [[Addison's disease]] is a case of abnormally low quantities of those hormones are produced by the adrenal gland, and it can be fatal. |
==Overview== | ==Overview== | ||
| Line 38: | Line 38: | ||
Cortisone is synthesized from [[cholesterol]] in the adrenal cortex via the stimulation of adrenocorticotropin hormone (ACTH) (Gramene 2007). | Cortisone is synthesized from [[cholesterol]] in the adrenal cortex via the stimulation of adrenocorticotropin hormone (ACTH) (Gramene 2007). | ||
| − | Cortisone is the inactive precursor molecule of the active hormone [[cortisol]], the "stress hormone." It is activated through | + | Cortisone is the inactive precursor molecule of the active hormone [[cortisol]], the "[[stress (medical)|stress]] hormone." It is activated through hydroxylation of the 11-keto-group by an [[enzyme]] called 11-beta-steroid dehydrogenase. The active form cortisol is thus sometimes referred to as [[hydrocortisone]]. |
| − | Both cortisone and cortisol are classified as glucocorticoids, a group of corticosteroids that controls protein, fat, carbohydrate, and calcium metabolism. (Minealocorticoids, the other group of corticosteroids, regulates salt and potassium levels and water retention.) Another hormone produced in the [[adrenal gland]]s, albeit in the adrenal medulla, not the adrenal cortex, is adrenaline (epinephrine), which like | + | Both cortisone and cortisol are classified as glucocorticoids, a group of corticosteroids that controls [[protein]], [[fat]], [[carbohydrate]], and [[calcium]] [[metabolism]]. (Minealocorticoids, the other group of corticosteroids, regulates salt and potassium levels and water retention.) Another hormone produced in the [[adrenal gland]]s, albeit in the adrenal medulla, not the adrenal cortex like corticosteroids, is [[adrenaline]] (epinephrine), which like cortisol, deals with stress. |
| − | Cortisone is involved in carbohydrate metablolism, with such effects listed as increased glucose release from the liver, increased liver glycogen synthesis, and decreased utilization of glucose by the tissues (Gramene 2007). | + | Cortisone is involved in carbohydrate metablolism, with such effects listed as increased [[glucose]] release from the [[liver]], increased liver [[glycogen]] synthesis, and decreased utilization of glucose by the tissues (Gramene 2007). Cortisone also stimulates protein catabolism and decreased protein synthesis. Goodlad and Munro (1958) noted that administred cortisone results in loss of protein from the body and accumulation of glycogen and protein in the liver. Cortisol also effects salt retention in the [[kidney]]s. |
| + | Cortisone is considered to be less important than cortisol. Cortisol is responsible for 95% of the effects of the glucocorticosteroids. while cortisone is about 4 or 5%. | ||
| + | |||
| + | Cortisone was first isolated by the American chemist Edward Calvin Kendall. He won the 1950 Nobel Prize for Physiology or Medicine along with Philip S. Hench and Tadeus Reichstein for the discovery of [[adrenal gland|adrenal cortex]] hormones, their structures, and functions. It was discovered that it could treat arthritis and was named "cortisone" on July 1, 1949. | ||
==Medical use== | ==Medical use== | ||
| Line 49: | Line 52: | ||
Cortisone is sometimes used as a drug to treat a variety of ailments. It can be administered [[intravenous]]ly or [[cutaneous]]ly. | Cortisone is sometimes used as a drug to treat a variety of ailments. It can be administered [[intravenous]]ly or [[cutaneous]]ly. | ||
| − | + | Cortisone has an anti-inflammatory effect in the short-term, and thus has been found helpful in treating rheumatoid [[arthritis]]. It also has been used to treat [[allergy|allergies]], dematology diseases, respiratory diseases, and endocrine disorders. It can be one of the most effective treatments for people with severe allergies. Cortisone can help in dissolving scar tissue and speed the healing process. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | One of cortisone's effects on the body, and a potentially harmful side effect when administered clinically, is the suppression of the [[immune system]]. Thus, [[bacteria]]l infection can be increased. This is one explanation for the apparent correlation between high [[Stress (medicine)|stress]] and sickness. On the other hand, the ability of cortisone to minimize the immune reaction has helped in terms of organ transplants. Some other side effects are peptic ulsers, depression, and even mood swings and insomnia. | ||
| + | Cortisone has been an effective medical tool. However, other drugs that are more effective, longer lasting, and have less side effects have largely replaced cortisone in medical treatment. | ||
==References== | ==References== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | * Goodlad, G. A. J., and H. N. Munro. 1959. Diet and the action of cortione on protein metabolism. ''Biochem J.'' 73(2): 343–348. | ||
| + | * Gramene. 2007. [http://www.gramene.org/db/ontology/search?query=carbohydrate%20metabolism&ontology_type=GO Summary for carbohydrate metabolism]. ''Gramene website''. Retrieved November 9, 2007. | ||
| + | * Ingle, D. J. 1950. The biologic properties of cortisone: A review. ''Journal of Clinical Endocrinology'' 10: 1312-1354. | ||
| + | * Merck. 1989. ''Merck Index'', 11th Edition. Merck & Company. ISBN 091191028X | ||
| + | * Woodward R. B., F. Sondheimer, and D. Taub. 1951. The total synthesis of cortisone. ''Journal of the American Chemical Society'' 73: 4057. | ||
{{credit|100857154}} | {{credit|100857154}} | ||
[[Category:Life sciences]] | [[Category:Life sciences]] | ||
Revision as of 04:46, 10 February 2007
| Cortisone | |
|---|---|
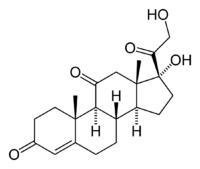
| |
| Systematic name | 17,21-dihydroxypregn-4-ene-3,11,20-trione |
| Chemical formula | C21H28O5 |
| Molecular mass | 360.46 g/mol |
| Density | ? g/cm3 |
| Melting point | 220-224 °C |
| CAS number | [53-06-5] |
| SMILES | C[C@@](C3)4[C@](CC[C@@](O)4 [C@@](CO)=O)([H])[C@]2([H])CCC1=CC (CC[C@@](C)1[C@]([H])2C3=O)=O |
| Disclaimer and references | |
Cortisone (17-hydroxy-11-dehydrocorticosterone) is a naturally occuring steroid hormone that functions in carbohydrate metabolism and is used medically for the treatment of various ailments, including rheumatoid arthritis and certain allergies. Its formula is formula C21H28O5 and IUPAC name is 17,21-dihydroxypregn-4-ene-3,11,20-trione.
Cortisone is a corticosteroid, a term that refers to steroid hormones that are produced in the adrenal cortex of the body. Cortisone is essential for life. Addison's disease is a case of abnormally low quantities of those hormones are produced by the adrenal gland, and it can be fatal.
Overview
Cortisone is synthesized from cholesterol in the adrenal cortex via the stimulation of adrenocorticotropin hormone (ACTH) (Gramene 2007).
Cortisone is the inactive precursor molecule of the active hormone cortisol, the "stress hormone." It is activated through hydroxylation of the 11-keto-group by an enzyme called 11-beta-steroid dehydrogenase. The active form cortisol is thus sometimes referred to as hydrocortisone.
Both cortisone and cortisol are classified as glucocorticoids, a group of corticosteroids that controls protein, fat, carbohydrate, and calcium metabolism. (Minealocorticoids, the other group of corticosteroids, regulates salt and potassium levels and water retention.) Another hormone produced in the adrenal glands, albeit in the adrenal medulla, not the adrenal cortex like corticosteroids, is adrenaline (epinephrine), which like cortisol, deals with stress.
Cortisone is involved in carbohydrate metablolism, with such effects listed as increased glucose release from the liver, increased liver glycogen synthesis, and decreased utilization of glucose by the tissues (Gramene 2007). Cortisone also stimulates protein catabolism and decreased protein synthesis. Goodlad and Munro (1958) noted that administred cortisone results in loss of protein from the body and accumulation of glycogen and protein in the liver. Cortisol also effects salt retention in the kidneys.
Cortisone is considered to be less important than cortisol. Cortisol is responsible for 95% of the effects of the glucocorticosteroids. while cortisone is about 4 or 5%.
Cortisone was first isolated by the American chemist Edward Calvin Kendall. He won the 1950 Nobel Prize for Physiology or Medicine along with Philip S. Hench and Tadeus Reichstein for the discovery of adrenal cortex hormones, their structures, and functions. It was discovered that it could treat arthritis and was named "cortisone" on July 1, 1949.
Medical use
Cortisone is sometimes used as a drug to treat a variety of ailments. It can be administered intravenously or cutaneously.
Cortisone has an anti-inflammatory effect in the short-term, and thus has been found helpful in treating rheumatoid arthritis. It also has been used to treat allergies, dematology diseases, respiratory diseases, and endocrine disorders. It can be one of the most effective treatments for people with severe allergies. Cortisone can help in dissolving scar tissue and speed the healing process.
One of cortisone's effects on the body, and a potentially harmful side effect when administered clinically, is the suppression of the immune system. Thus, bacterial infection can be increased. This is one explanation for the apparent correlation between high stress and sickness. On the other hand, the ability of cortisone to minimize the immune reaction has helped in terms of organ transplants. Some other side effects are peptic ulsers, depression, and even mood swings and insomnia.
Cortisone has been an effective medical tool. However, other drugs that are more effective, longer lasting, and have less side effects have largely replaced cortisone in medical treatment.
ReferencesISBN links support NWE through referral fees
- Goodlad, G. A. J., and H. N. Munro. 1959. Diet and the action of cortione on protein metabolism. Biochem J. 73(2): 343–348.
- Gramene. 2007. Summary for carbohydrate metabolism. Gramene website. Retrieved November 9, 2007.
- Ingle, D. J. 1950. The biologic properties of cortisone: A review. Journal of Clinical Endocrinology 10: 1312-1354.
- Merck. 1989. Merck Index, 11th Edition. Merck & Company. ISBN 091191028X
- Woodward R. B., F. Sondheimer, and D. Taub. 1951. The total synthesis of cortisone. Journal of the American Chemical Society 73: 4057.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.