Difference between revisions of "Chemical decomposition" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
(added credit and category tags, deleted foreign language links) |
||
| Line 53: | Line 53: | ||
*[http://umbbd.msi.umn.edu Biodegradation database] | *[http://umbbd.msi.umn.edu Biodegradation database] | ||
| + | [[Category:Physical sciences]] | ||
| + | [[Category:Chemistry]] | ||
[[Category:Inorganic chemistry]] | [[Category:Inorganic chemistry]] | ||
[[Category:Organic chemistry]] | [[Category:Organic chemistry]] | ||
| − | |||
| − | |||
| − | + | {{credit|250471842}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 16:09, 9 December 2008
Chemical decomposition or analysis is the separation of a chemical compound into elements or smaller compounds. It is sometimes defined as the opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction. The stability that a chemical compound ordinarily has is eventually limited when exposed to extreme environmental conditions like heat, radiation, humidity or the acidity of a solvent. The details of decomposition processes are generally not well defined, as a molecule may break up into a host of smaller fragments. Chemical decomposition is exploited in several analytical techniques, notably mass spectrometry, traditional gravimetric analysis, and thermogravimetric analysis.
A broader definition of the term decomposition also includes the breakdown of one phase into two or more phases.[1]
There are broadly 3 types of decomposition reactions: thermal,elecrolytic and catalytic.
Reaction formulas
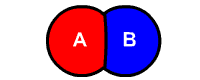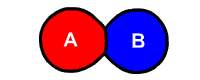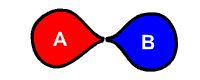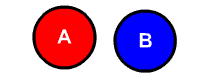
The generalized reaction formula for chemical decomposition is:
- AB → A + B
with a specific example being the electrolysis of water to gaseous hydrogen and oxygen:
- 2H2O → 2H2 + O2
Additional examples
An example of spontaneous decomposition is that of hydrogen peroxide, which will slowly decompose into water and oxygen:
- 2H2O2 → 2H2O + O2
Carbonates will decompose when heated, a notable exception being that of carbonic acid, H2CO3. Carbonic acid, the "fizz" in sodas, pop cans and other carbonated beverages, will decompose over time (spontaneously) into carbon dioxide and water
- H2CO3 → H2O + CO2
Other carbonates will decompose when heated producing the corresponding metal oxide and carbon dioxide. In the following equation M represents a metal:
- MCO3 → MO + CO2
A specific example of this involving calcium carbonate:
- CaCO3 → CaO + CO2
Metal chlorates also decompose when heated. A metal chloride and oxygen gas are the products.
- MClO3 → MCl + O2
A common decomposition of a chlorate to evolve oxygen utilizes potassium chlorate as follows:
- 2KClO3 → 2KCl + 3O2
See also
- Analytical chemistry
- Thermal decomposition
ReferencesISBN links support NWE through referral fees
- ↑ International Union of Pure and Applied Chemistry. "decomposition". Compendium of Chemical Terminology Internet edition.
External links
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.