Charles-Augustin de Coulomb
<<Please summarize the significance of Coulomb's life in the introductory paragraph.>>
Charles-Augustin de Coulomb (June 14, 1736 – August 23, 1806) was a French physicist.
Biography
<<We need more information in his biography. Some details of his scientific accomplishments can be placed in a separate section(s), as done below.>>
Coulomb was born in Angoulême, France. He chose the profession of military engineer, and spent three years, to the decided injury of his health, at Fort Bourbon, Martinique. Upon his return, he was employed at La Rochelle, the Isle of Aix and Cherbourg. He discovered an inverse relationship on the force between charges and the square of its distance, later named after him as Coulomb's law.
In 1781, he was stationed permanently at Paris. On the outbreak of the Revolution in 1789, he resigned his appointment as intendant des eaux et fontaines, and retired to a small estate which he possessed at Blois. He was recalled to Paris for a time in order to take part in the new determination of weights and measures, which had been decreed by the Revolutionary government. Of the National Institute he was one of the first members; and he was appointed inspector of public instruction in 1802. But his health was already very feeble, and four years later he died in Paris.
Scientific accomplishments
Coulomb is distinguished in the history of mechanics and of electricity and magnetism. In 1779, he published an important investigation of the laws of friction (Théorie des machines simples, en ayant égard au frottement de leurs parties et à la roideur des cordages), which was followed twenty years later by a memoir on viscosity.
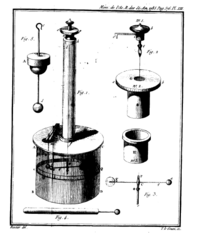
In 1784 his Recherches théoriques et expérimentales sur la force de torsion et sur l'élasticité des fils de metal (Histoire de l’Académie Royale des Sciences, 229-269, 1784) appeared. This memoir contained a description of different forms of his torsion balance. He used the instrument with great success for the experimental investigation of the distribution of charge on surfaces, of the laws of electrical and magnetic force, and of the mathematical theory of which he may also be regarded as the founder.
In 1785 Coulomb presented his three reports on Electricity and Magnetism:
- Premier Mémoire sur l’Electricité et le Magnétisme, Histoire de l’Académie Royale des Sciences, 569-577, 1785. In this publication Coulomb describes “How to construct and use an electric balance (torsion balance) based on the property of the metal wires of having a reaction torsion force proportional to the torsion angle.” Coulomb also determinates experimentally the law that explains how “two bodies electrified of the same kind of Electricity exert on each other.”
- Sécond Mémoire sur l’Electricité et le Magnétisme, Histoire de l’Académie Royale des Sciences, 578-611, 1785. In this publication Coulomb carries out the “determination according to which laws both the Magnetic and the Electric fluids act, either by repulsion or by attraction.”
- Troisième Mémoire sur l’Electricité et le Magnétisme, Histoire de l’Académie Royale des Sciences, 612-638, 1785. “On the quantity of Electricity that an isolated body losses in a certain time period , either by contact with less humid air, or in the supports more or less idio-electric.”
Coulomb explained the laws of attraction and repulsion between electric charges and magnetic poles, although he did not find any relationship between the two phenomena. He thought that the attraction and repulsion were due to different kinds of fluids.
The SI unit of charge, the coulomb, and Coulomb's law are named after him.
Coulomb's law
Using a torsion balance, Coulomb was able to measure the electrostatic force between two electrically charged objects of small dimensions. His observations led him to discover a mathematical relationship that came to be called Coulomb's law. This law may be stated as follows:
The magnitude of the electrostatic force between two point charges is directly proportional to the magnitudes of each charge and inversely proportional to the square of the distance between the charges.
This is analogous to Newton's third law of motion in mechanics. The formula to Coulomb's Law is of the same form as Newton's Gravitational Law: The electrical force of one body exerted on the second body is equal to the force exerted by the second body on the first.
Scalar form of the law:
To calculate the magnitude of the force (and not in its direction), it may be easiest to consider the simplified, scalar version of the law:
where:
- is the magnitude of the force exerted,
- is the charge on one body,
- is the charge on the other body,
- is the distance between them,
- 8.988×109 N m2 C-2 (also m F-1) is the electrostatic constant or Coulomb force constant, and
- 8.854×10−12 C2 N-1 m-2 (also F m-1) is the permittivity of free space, also called electric constant, an important physical constant.
In cgs units, the unit charge, esu of charge or statcoulomb, is defined so that this Coulomb force constant is 1.
The force acts on the line connecting the two charged objects. Charged objects of the same polarity repel each other along this line and charged objects of opposite polarity attract each other along this line.
When measured in units that people commonly use (such as MKS - see International System of Units), the Coulomb force constant, , is numerically much much larger than the universal gravitational constant . This means that for objects with charge that is of the order of a unit charge (C) and mass of the order of a unit mass (kg), the electrostatic forces will be so much larger than the gravitational forces that the latter force can be ignored. This is not the case when Planck units are used and both charge and mass are of the order of the unit charge and unit mass. However, charged elementary particles have mass that is far less than the Planck mass while their charge is about the Planck charge so that, again, gravitational forces can be ignored.
See also
ReferencesISBN links support NWE through referral fees
<<We need at least 3 reliable references here, properly formatted.>>
External links
- French National Library The Mémoires of Coulomb available in pdf format.
- John J. O'Connor and Edmund F. Robertson. Charles-Augustin de Coulomb at the MacTutor archive
This article incorporates text from the Encyclopædia Britannica Eleventh Edition, a publication now in the public domain.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.