Difference between revisions of "Catecholamine" - New World Encyclopedia
({{Contracted}}) |
Rick Swarts (talk | contribs) |
||
| Line 1: | Line 1: | ||
{{Contracted}} | {{Contracted}} | ||
| − | |||
[[Image:Adrenaline.svg|thumb|[[epinephrine]]]] | [[Image:Adrenaline.svg|thumb|[[epinephrine]]]] | ||
[[Image:Norepinephrine structure.png|thumb|[[norepinephrine]]]] | [[Image:Norepinephrine structure.png|thumb|[[norepinephrine]]]] | ||
[[Image:Dopamine.png|thumb|[[dopamine]]]] | [[Image:Dopamine.png|thumb|[[dopamine]]]] | ||
| + | [[Image:L-tyrosine-skeletal.png|thumb|[[tyrosine]] is the precursor of catecholamines]] | ||
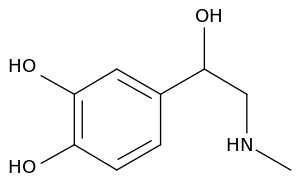
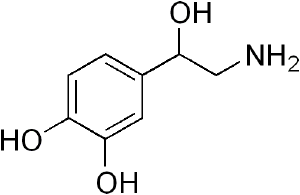
| + | '''Catecholamine''' is any of a group of amines (nitrogen-contain organic compounds) derived from the [[amino acid]] [[tyrosine]] and containing a [[catechol]] group (aromatic chemical compound consisting of a benzene ring with two hydroxyl groups). Catecholamine are important as [[neurotransmitter]]s and [[hormone]]s. The most abundant catecholamines are [[epinephrine]] (adrenaline), [[norepinephrine]] (noradrenaline) and [[dopamine]], all of which are produced by phenylalanine and tyrosine. | ||
| + | |||
| + | Catecholamines as [[hormone]] are released by the [[adrenal gland]]s in situations of [[stress (medical)|stress] such as [[Fight-or-flight response|psychological stress]] or [[low blood sugar]] levels (Hoffman 1999). | ||
| + | |||
| + | ==Structure== | ||
| + | Catecholamines are [[chemical compounds]] that are derived from [[tyrosine]] and contain both a catechol group and an amine group. | ||
| + | |||
| + | [[Image:Pyrocatechol.svg|thumb|left|Catechol]] | ||
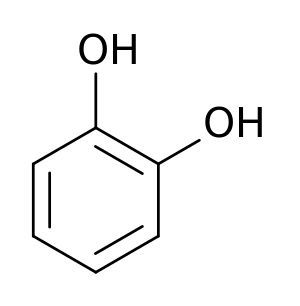
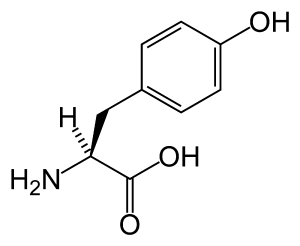
| + | Tyrosine is an α-[[amino acid]] that is found in most [[protein]]s and is normally readily converted from the essential amino acid [[phenylalanine]] in the [[human body]]. Tyrosine's chemical formula is C<sub>9</sub>H<sub>11</sub>NO<sub>3</sub> (IUPAC-IUB 1983) (that is, one more [[nitrogen]] atom than phenylalanine) and it contains a large rigid aromatic group on the side chain—a [[phenol]] side chain with a [[hydroxyl]] group. Catechol or pyrocatechol is a [[benzenediol]] (aromatic chemical compounds in which two hydroxyl groups are substituted onto a benzene ring) with the formula C<sub>6</sub>H<sub>4</sub>(OH)<sub>2</sub>. An amine group is a functional group that contain [[nitrogen]] as the key atom. Thus, a catecolamine has the distinct structure of a [[benzene ring]] with two [[hydroxyl]] groups, an intermediate [[ethyl]] chain, and a terminal [[amine]] group. Some of them are [[biogenic amine]]s (substances produced by life processes). | ||
| + | |||
| + | Catecholamines are water soluble and are 50% bound to plasma proteins, so they circulate in the bloodstream. | ||
| + | |||
| + | ==Production== | ||
[[Image:Biosynthese Adrenalin.png|thumb|Synthesis]] | [[Image:Biosynthese Adrenalin.png|thumb|Synthesis]] | ||
| − | + | Catecholamines are produced mainly by the [[chromaffin cells]] of the [[adrenal gland|adrenal medulla]] and the [[postganglionic fiber]]s of the [[autonomic nervous system|sympathetic nervous system]]. Epinephrine (or [[adrenaline]]) and norepinephrine ([[noradrenaline]]) are [[hormone]]s that are secreted principally by the adrenal medulla. The [[adrenal gland]], located atop the [[kidney]]s, is separated into two distinct structures, the adrenal medulla and the adrenal cortex. The adrenal medulla is at the center of the adrenal gland and is surrounded by the adrenal cortex, with the adrenal medulla taking up about one-quarter of the adrenal gland and the adrenal cortex the remaining three-quarters. The adrenal glands are chiefly responsible for regulating the [[stress (medicine)|stress]] response through the synthesis of [[corticosteroid]]s and catecholamines. | |
| + | |||
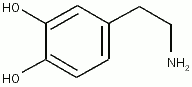
| + | [[Dopamine]], which acts as a [[neurotransmitter]] in the [[central nervous system]], is largely produced in neuronal cell bodies in two areas of the brainstem: the [[substantia nigra]] and the [[ventral tegmental area]]. | ||
| + | |||
| + | Tryosine, one of the main precursors of catecholamine, is created from [[phenylalanine]] by hydroxylation via the [[enzyme]] phenylalanine hydroxylase. (Tyrosine is also ingested directly from dietary protein). Tyrosine is then sent to catecholaminesecreting [[neuron]]s. Here many kinds of reactions convert it to dopamine, and eventually to norepinephrine and epinephrine (Joh and Hwang 1987). | ||
| + | [[Image:Biosynthese Adrenalin.png|thumb|300px|Epinephrine is synthesized from [[norepinephrine]] in a synthetic pathway shared by all catecholamines.]] | ||
| + | |||
| + | Norepinephrine is synthesized by a series of enzymatic steps in the adrenal medulla from the amino acid tyrosine: | ||
| + | * The first reaction is the oxidation into dihydroxyphenylalanine (L-DOPA). | ||
| + | * This is followed by decarboxylation into the neurotransmitter dopamine. | ||
| + | * Last is the final β-oxidation into norepinephrine by dopamine beta hydroxylase. | ||
| + | |||
| + | |||
| + | Epinephrine is synthesized from [[norepinephrine]] in a synthetic pathway shared by all catecholamines, including [[L-dopa]], [[dopamine]], [[norepinephrine]], and epinephrine. | ||
| + | |||
| + | Epinephrine is synthesized via methylation of the primary distal amine of norepinephrine by phenylethanolamine N-methyltransferase (PNMT) in the [[cytoplasm|cytosol]] of adrenergic neurons and cells of the [[adrenal gland|adrenal medulla]] (so-called chromaffin cells). PNMT is only found in the cytosol of cells of adrenal medullary cells. PNMT uses ''S''-adenosylmethionine (SAMe) as a cofactor to donate the [[methyl]] group to norepinephrine, creating epinephrine. | ||
| + | |||
| + | For norepinephrine to be acted upon by PNMT in the cytosol, it must first be shipped out of granules of the chromaffin cells. This may occur via the catecholamine-H<sup>+</sup> exchanger VMAT1. VMAT1 is also responsible for transporting newly synthesized epinephrine from the cytosol back into chromaffin granules in preparation for release. | ||
| − | |||
| − | |||
==Function== | ==Function== | ||
| Line 21: | Line 49: | ||
Catecholamines cause general physiological changes that prepare the body for physical activity ([[fight-or-flight response]]). Some typical effects are increases in heart rate, [[blood pressure]], [[blood glucose]] levels, and a general reaction of the [[sympathetic nervous system]]. Some drugs, like [[tolcapone]] (a central [[COMT]]-inhibitor), raise the levels of all the catecholamines. | Catecholamines cause general physiological changes that prepare the body for physical activity ([[fight-or-flight response]]). Some typical effects are increases in heart rate, [[blood pressure]], [[blood glucose]] levels, and a general reaction of the [[sympathetic nervous system]]. Some drugs, like [[tolcapone]] (a central [[COMT]]-inhibitor), raise the levels of all the catecholamines. | ||
| − | |||
| − | |||
==Degradation== | ==Degradation== | ||
| Line 43: | Line 69: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | |||
| + | <ref>[http://www.consciouschoice.com/1999/cc1207/hmd1207.html "Hypoglycemia"] by Ronald Hoffman, M.D., July 1999, The Holistic M.D.</ref>. | ||
| + | |||
| + | |||
| + | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | ||
| + | |||
| + | <ref>[http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=PubMed&list_uids=3473965&dopt=Abstract]</ref> | ||
| + | |||
| + | 1: Ann N Y Acad Sci. 1987;493:342-50. Related Articles, Links | ||
| + | Dopamine beta-hydroxylase: biochemistry and molecular biology. | ||
| + | Joh TH, Hwang O. | ||
| + | |||
==External links== | ==External links== | ||
Revision as of 15:58, 2 August 2007
Catecholamine is any of a group of amines (nitrogen-contain organic compounds) derived from the amino acid tyrosine and containing a catechol group (aromatic chemical compound consisting of a benzene ring with two hydroxyl groups). Catecholamine are important as neurotransmitters and hormones. The most abundant catecholamines are epinephrine (adrenaline), norepinephrine (noradrenaline) and dopamine, all of which are produced by phenylalanine and tyrosine.
Catecholamines as hormone are released by the adrenal glands in situations of [[stress (medical)|stress] such as psychological stress or low blood sugar levels (Hoffman 1999).
Structure
Catecholamines are chemical compounds that are derived from tyrosine and contain both a catechol group and an amine group.
Tyrosine is an α-amino acid that is found in most proteins and is normally readily converted from the essential amino acid phenylalanine in the human body. Tyrosine's chemical formula is C9H11NO3 (IUPAC-IUB 1983) (that is, one more nitrogen atom than phenylalanine) and it contains a large rigid aromatic group on the side chain—a phenol side chain with a hydroxyl group. Catechol or pyrocatechol is a benzenediol (aromatic chemical compounds in which two hydroxyl groups are substituted onto a benzene ring) with the formula C6H4(OH)2. An amine group is a functional group that contain nitrogen as the key atom. Thus, a catecolamine has the distinct structure of a benzene ring with two hydroxyl groups, an intermediate ethyl chain, and a terminal amine group. Some of them are biogenic amines (substances produced by life processes).
Catecholamines are water soluble and are 50% bound to plasma proteins, so they circulate in the bloodstream.
Production
Catecholamines are produced mainly by the chromaffin cells of the adrenal medulla and the postganglionic fibers of the sympathetic nervous system. Epinephrine (or adrenaline) and norepinephrine (noradrenaline) are hormones that are secreted principally by the adrenal medulla. The adrenal gland, located atop the kidneys, is separated into two distinct structures, the adrenal medulla and the adrenal cortex. The adrenal medulla is at the center of the adrenal gland and is surrounded by the adrenal cortex, with the adrenal medulla taking up about one-quarter of the adrenal gland and the adrenal cortex the remaining three-quarters. The adrenal glands are chiefly responsible for regulating the stress response through the synthesis of corticosteroids and catecholamines.
Dopamine, which acts as a neurotransmitter in the central nervous system, is largely produced in neuronal cell bodies in two areas of the brainstem: the substantia nigra and the ventral tegmental area.
Tryosine, one of the main precursors of catecholamine, is created from phenylalanine by hydroxylation via the enzyme phenylalanine hydroxylase. (Tyrosine is also ingested directly from dietary protein). Tyrosine is then sent to catecholaminesecreting neurons. Here many kinds of reactions convert it to dopamine, and eventually to norepinephrine and epinephrine (Joh and Hwang 1987).
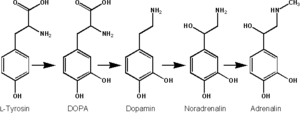
Norepinephrine is synthesized by a series of enzymatic steps in the adrenal medulla from the amino acid tyrosine:
- The first reaction is the oxidation into dihydroxyphenylalanine (L-DOPA).
- This is followed by decarboxylation into the neurotransmitter dopamine.
- Last is the final β-oxidation into norepinephrine by dopamine beta hydroxylase.
Epinephrine is synthesized from norepinephrine in a synthetic pathway shared by all catecholamines, including L-dopa, dopamine, norepinephrine, and epinephrine.
Epinephrine is synthesized via methylation of the primary distal amine of norepinephrine by phenylethanolamine N-methyltransferase (PNMT) in the cytosol of adrenergic neurons and cells of the adrenal medulla (so-called chromaffin cells). PNMT is only found in the cytosol of cells of adrenal medullary cells. PNMT uses S-adenosylmethionine (SAMe) as a cofactor to donate the methyl group to norepinephrine, creating epinephrine.
For norepinephrine to be acted upon by PNMT in the cytosol, it must first be shipped out of granules of the chromaffin cells. This may occur via the catecholamine-H+ exchanger VMAT1. VMAT1 is also responsible for transporting newly synthesized epinephrine from the cytosol back into chromaffin granules in preparation for release.
Function
Modality
Two catecholamines, norepinephrine and dopamine, act as neurotransmitters in the central nervous system and as hormones in the blood circulation. The catecholamine norepinephrine is a neurotransmitter of the peripheral sympathetic nervous system but is also present in the blood (mostly through "spillover" from the synapses of the sympathetic system).
High catecholamine levels in blood are associated with stress, which can be induced from psychological reactions or environmental stressors such as elevated sound levels, intense light, or low blood sugar levels.
Extremely high levels of catecholamine (also known as catecholamine toxicity) can occur in CNS trauma due to stimulation and/or damage of nuclei in the brainstem, particularly those nuclei affecting the sympathetic nervous system. In emergency medicine, this occurrence is widely known as catecholamine dump.
Effects
Catecholamines cause general physiological changes that prepare the body for physical activity (fight-or-flight response). Some typical effects are increases in heart rate, blood pressure, blood glucose levels, and a general reaction of the sympathetic nervous system. Some drugs, like tolcapone (a central COMT-inhibitor), raise the levels of all the catecholamines.
Degradation
They have a half-life of approximately a few minutes when circulating in the blood.
Monoamine oxidase (MAO) is the main enzyme responsible for degradation of catecholamines.
Methamphetamine and MAOIs bind to MAOs to inhibit their action of breaking down catecholamines. This is primarily the reason why the effects of amphetamines last longer than cocaine and other substances. Amphetamines not only causes a release of dopamine, epinephrine, and norepinephrine into the blood stream, but also keeps it working there for a long time.
See also
- Catechol-O-methyl transferase
- Hormone
- Julius Axelrod
- Phenethylamines
- Steroid hormone
- Peptide hormone
- Sympathomimetics
- Vanillyl mandelic acid
ReferencesISBN links support NWE through referral fees
[1].
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
1: Ann N Y Acad Sci. 1987;493:342-50. Related Articles, Links Dopamine beta-hydroxylase: biochemistry and molecular biology. Joh TH, Hwang O.
External links
- MeSH Catecholamines
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ "Hypoglycemia" by Ronald Hoffman, M.D., July 1999, The Holistic M.D.
- ↑ [1]