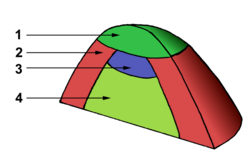
Meristem
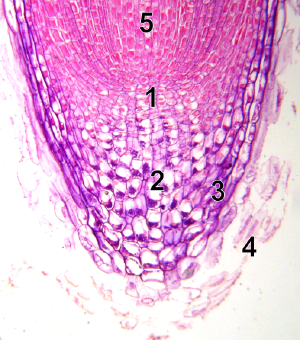
Meristem is a region of plant tissue consisting of undifferentiated or incompletely differentiated cells (meristematic cells) that are capable of cell division and growth and from which new cells are formed.
Differentiated plant cells generally cannot divide or produce cells of a different type. Therefore, cell division in the meristem is required to provide new cells for expansion and differentiation of tissues and initiation of new organs, providing the basic structure of the plant body. These meristematic cells are analogous in function to stem cells in animals.
Maintenance of the cells requires a balance between two antagonistic processes: organ initiation and stem cell population renewal. They have both the ability to renew themselves through mitotic cell division and the ability to differentiate into a diverse range of specialized cell types.
Overview
Cellular differentiation is the process by which a less specialized cell becomes a more specialized cell type. Differentiation occurs numerous times during the development of a multicellular organism as the organism changes from a single zygote to a complex system of tissues and cell types. A cell that is able to differentiate into many cell types is known as pluripotent. These cells are called stem cells in animals and meristematic cells in higher plants.
Meristematic cells are incompletely or not at all differentiated, and are capable of continued cellular division (youthful). Furthermore, the cells are small and protoplasm fills the cell completely. The vacuoles are extremely small. The cytoplasm does not contain differentiated plastids (chloroplasts or chromoplasts), although they are present in rudimentary form (proplastids). Meristematic cells are packed closely together without intercellular cavities. The cell wall is a very thin primary cell wall.
The term ‚Äúmeristem‚ÄĚ was first used by Karl Wilhelm von N√§geli (1817-1891) from his book ‚ÄúBeitr√§ge zur Wissenschaftlichen Botanik‚ÄĚ in 1858. It is derived from the Greek word ‚Äúmerizein,‚ÄĚ meaning to divide in recognition of its inherent function.
Meristematic zones
Apical meristems are the completely undifferentiated (indeterminate) meristems in a plant. These differentiate into three kinds of primary meristems. The primary meristems in turn produce the two secondary meristem types. These secondary meristems are also known as lateral meristems because they are involved in lateral growth.
At the meristem summit there is a small group of slowly dividing cells that is commonly called the central zone. Cells of this zone have a stem cell function and are essential for meristem maintenance. The proliferation and growth rates at the meristem summit usually differ considerably from those at the periphery.
Primary meristems
Apical meristems may differentiate into three kinds of primary meristem:
- Protoderm‚ÄĒlies around the outside of the stem and develops into the epidermis.
- Procambium‚ÄĒlies just inside of the protoderm and develops into primary xylem and primary phloem. It also produces the vascular cambium, a secondary meristem.
- Ground meristem‚ÄĒdevelops into the pith. It produces the cork cambium, another secondary meristem.
These meristems are responsible for primary growth, or an increase in length or height.
Secondary meristems
There are two types of secondary meristems. These are also called the lateral meristems because they surround the established stem of a plant and cause it to grow laterally (that is, larger in diameter).
- Vascular cambium‚ÄĒproduces secondary xylem and secondary phloem. This is a process that may continue throughout the life of the plant. This is what gives rise to wood in plants. Such plants are called arborescent. This does not occur in plants that do not go through secondary growth (known as herbaceous plants).
- Cork cambium‚ÄĒa lateral meristem that is responsible for secondary growth that replaces the epidermis in roots and stems. It is found in woody and many herbaceous dicots, gymnosperms and some monocots, which usually lack secondary growth. Cork cambium is one of the many layers of bark, between the cork and primary phloem. The function of cork cambium is to produce the cork, a tough protective material.
Basal meristems
As the name implies, this type of meristem is not found at the tip of a root or shoot, but near the base. This type of meristem allows for primary growth even after the apex of the shoot has been severed. For example, the presence of basal meristem is the reason grass can continue growing after mowing.
Apical meristems
The apical meristem, or growing tip, is a completely undifferentiated meristematic tissue found in the buds and growing tips of roots in plants. Its main function is to begin growth of new cells in young seedlings at the tips of roots and shoots (forming buds, among other structures). Specifically, an active apical meristem lays down a growing root or shoot behind itself, pushing itself forward. Apical meristems are very small, compared to the cylinder-shaped lateral meristems.
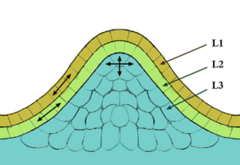
Apical meristems are composed of several layers. The number of layers varies according to plant type. In general, the outermost layer is called the tunica while the innermost layers are the corpus. In monocots, the tunica determine the physical characteristics of the leaf edge and margin. In dicots, layer two of the corpus determine the characteristics of the edge of the leaf. The corpus and tunica play a critical part of the plant physical appearance as all plant cells are formed from the meristems. Apical meristems are found in two locations: The root and the stem. Some Arctic plants have a apical meristem in the lower/middle parts of the plant.
Shoot apical meristems
The source of all above-ground organs are shoot apical meristems (SAM). Cells at the SAM summit serve as stem cells to the surrounding peripheral region, where they proliferate rapidly and are incorporated into differentiating leaf or flower primordia.
The shoot apical meristem is the site of most of the embryogenesis in flowering plants. Primordia of leaves, sepals, petals, stamens, and ovaries are initiated here at the rate of one every time interval, called a plastochron. It is where the first indications that flower development has been evoked are manifested. One of these indications might be the loss of apical dominance and the release of otherwise dormant cells to develop as axillary shoot meristems‚ÄĒin some species in axils of primordia as close as two or three away from the apical dome. The SAM consists of 4 distinct cell groups:
- Stem cells
- The immediate daughter cells of the stem cells
- A subjacent organizing center
- Founder cells for organ initiation in surrounding regions
The four distinct zones mentioned above are maintained by a complex signaling pathway. In Arabidopsis thaliana, three interacting CLAVATA genes are required to regulate the size of the stem cell reservoir in the SAM by controlling the rate of cell division (Fletcher 2002). CLV1 and CLV2 are predicted to form a receptor complex (of the LRR receptor like kinase family) to which CLV3 is a ligand (Clark et al. 1997; Jeong et al. 1999; Fletcher et al. 1999). CLV3 shares some homology with the ESR proteins of maize, with a short 14 amino acid region being conserved between the proteins (Cock and McCormick 2001; Oelkers et al. 2008). Proteins that contain these conserved regions have been grouped into the CLE family of proteins (Cock and McCormick 2001; Oelkers et al. 2008).
CLV1 has been shown to interact with several cytoplasmic proteins that are most likely involved in downstream signaling; for example, the CLV complex has been found to be associated with Rho/Rac small GTPase related proteins (Fletcher 2002). These proteins may act as an intermediate between the CLV complex and a mitogen-activated protein kinase (MAPK) that is often involved in signaling cascades (Valster et al. 2000). KAPP is a kinase-associated protein phosphatase that has been shown to interact with CLV1 (Stone et al. 1998). KAPP is thought to act as a negative regulator of CLV1 by dephosphorylating it (Stone et al. 1998).
Another important gene in plant meristem maintenance is WUSCHEL (shortened to WUS), which is a target of CLV signaling (Mayer et al. 1998). WUS is expressed in the cells below the stem cells of the meristem and its presence prevents the differentiation of the stem cells (Mayer et al. 1998). CLV1 acts to promote cellular differentiation by repressing WUS activity outside of the central zone containing the stem cells (Mayer et al. 1998). STM also acts to prevent the differentiation of stem cells by repressing the expression of Myb genes that are involved in cellular differentiation (Fletcher 2002).
Root apical meristems
Unlike the SAM, the root apical meristem (RAM) produces cells in two directions. It is covered by the root cap, which protects the apical meristem from the rocks, dirt, and pathogens. Cells are continuously sloughed off the outer surface of the root cap. The center of the RAM is occupied by a quiescent center, which has low mitotic activity. Evidence suggests the quiescent center does function as the zone of initials. Infrequent division of initial cells in the quiescent center is the source of cells for the RAM. These initial cells and tissue patterns become established in the embryo in the case of the primary root and in the new lateral meristems in the case of secondary roots.
Intercalary meristem
In angiosperms, intercalary meristems occur only in monocot (particularly grass) stems at the base of nodes and leaf blades. Horsetails also exhibit intercalary growth. Intercalary meristems are capable of cell division and allow for rapid growth and regrowth of many monocots. Intercalary meristems at the nodes of bamboo allow for rapid stem elongation, while those at the base of most grass leaf blades allow damaged leaves to rapidly regrow. This leaf regrowth in grasses evolved in response to damage by grazing herbivores, but is more familiar to many people in response to lawnmowers.
Floral meristem
When plants begin the developmental process known as flowering, the shoot apical meristem is transformed into an inflorescence meristem, which goes on to produce the floral meristem, which produces the familiar sepals, petals, stamens, and carpels of the flower.
In contrast to vegetative apical meristems and some exflorescence meristems, floral meristems are responsible for determinate growth, the limited growth of the flower to a particular size and form. The transition from shoot meristem to floral meristem requires floral meristem identity genes, that both specify the floral organs and cause the termination of the production of stem cells. AGAMOUS (AG) is a floral homeotic gene required for floral meristem termination and necessary for proper development of the stamens and carpels (Fletcher 2002). AG is necessary to prevent the conversion of floral meristems to inflorescence shoot meristems, but is not involved in the transition from shoot to floral meristem (Mizukami and Ma 1997). AG is turned on by the floral meristem identity gene LEAFY (LFY) and WUS and is restricted to the centre of the floral meristem or the inner two whorls (Lohmann et al. 2001). This way floral identity and region specificity is achieved. WUS activates AG by binding to a consensus sequence in the AG’s second intron and LFY binds to adjacent recognition sites (Mayer et al. 1998). Once AG is activated it represses expression of WUS leading to the termination of the meristem (Mayer et al. 1998).
Through the years scientists have manipulated floral meristems for economic reasons. An example is the mutant tobacco plant "Maryland Mammoth." In 1936, the department of agriculture of Switzerland performed several scientific tests with this plant. "Maryland Mammoth" is peculiar in this sense that it grows much faster than other tobacco plants.
Apical dominance
Apical dominance is the phenomenon where one meristem prevents or inhibits the growth of other meristems. As a result, the plant will have one clearly defined main trunk. For example, in trees the tip of the main trunk bears the dominant meristem. Therefore, the tip of the trunk grows fast and is not shadowed by branches. If the dominant meristem is cut off, one or more branch tips will assume dominance. The branch will start growing faster and the new growth will be vertical. Over the years the branch may begin to look more and more like an extension of the main trunk. Often several branches will exhibit this behavior after the removal of apical meristem, leading to a bushy growth.
The mechanism of apical dominance is based on the plant hormone auxin. It is produced in the apical meristem and transported towards the roots in the cambium. If apical dominance is complete, it prevents any branches from forming as long as apical meristem is active. If the dominance is incomplete, side branches will develop.
Indeterminate growth of meristems
Though each plant grows according to a certain set of rules, each new root and shoot meristem can go on growing for as long as it is alive. In many plants, meristematic growth is potentially indeterminate, making the overall shape of the plant not determinate in advance. This is the primary growth. Primary growth leads to lengthening of the plant body and organ formation. All plant organs arise ultimately from cell divisions in the apical meristems, followed by cell expansion and differentiation. Primary growth gives rise to the apical part of many plants.
Cloning
Under appropriate conditions, each shoot meristem can develop into a complete new plant or clone. Such new plants can be grown from shoot cuttings that contain an apical meristem. Root apical meristems are not readily cloned, however.
This cloning is called asexual reproduction or vegetative reproduction and is widely practiced in horticulture to mass-produce plants of a desirable genotype. This process is also known as mericloning.
ReferencesISBN links support NWE through referral fees
- Clark, S. E., R. W. Williams, and E. M. Meyerowitz. 1997. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89: 575‚Äď85.
- Cock, J. M., and S. McCormick. 2001. A large family of genes that share homology with CLAVATA3. Plant Physiology 126: 939‚Äď942. Retrieved November 5, 2008.
- Fletcher, J. C., U. Brand, M. P. Running, R. Simon, and E. M. Meyerowitz. 1999. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911‚Äď14.
- Fletcher, J. C. 2002. Shoot and floral meristem maintenance in Arabidopsis. Annu. Rev. Plant Biol. 53:45‚Äď66.
- Jeong, S., A. E. Trotochaud, and S. E. Clark. 1999. The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11: 1925‚Äď33.
- Lohmann, J. U. et al. 2001. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105: 793-803.
- Mayer, K. F. X. et al. 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805‚Äď815.
- Mizukami, Y., and H. Ma, H (1997) Determination of Arabidopsis floral meristem identity by AGAMOUS. The Plant Cell 9: 393- 408.
- Oelkers, K., N. Goffard, G. F. Weiller, P. M. Gresshoff, U. Mathesius, and T. Frickey. 2008. Bioinformatic analysis of the CLE signaling peptide family BMC. Plant Biology 8:1.
- Schoof, H., M. Lenhard, A. Haecker, K. F. Mayer, G. J√ľrgens, and T. Laux. 2000. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between CLAVATA and WUSCHEL genes. Cell 100: 635-644. Retrieved November 5, 2008.
- Scofield, S., and J. A. H. Murray. 2006. The evolving concept of the meristem. Plant Molecular Biology 60:v‚Äďvii. Retrieved November 5, 2008.
- Stone, J. M. et al. 1998. Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions. Plant Physiology 117: 1217-1225.
- Valster, A. H. et al. 2000. Plant GTPases: The Rhos in bloom. Trends in Cell Biology 10(4): 141-146.
| ||||||||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.