Emulsion
An emulsion is a mixture of two immiscible (unblendable) substances. One substance, called the "dispersed phase," is dispersed in the other, known as the "continuous phase." Examples of emulsions include butter, margarine, espresso, mayonnaise, the photosensitive side of photographic film, and cutting fluid for metalworking. The process of preparing emulsions is called emulsification.
Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, the word emulsion tends to imply that the dispersed and continuous phases are both liquids.
In the case of butter and margarine, a continuous lipid phase surrounds droplets of water—they are examples of "water-in-oil" emulsions. Mayonnaise is an "oil-in-water" emulsion stabilized with lecithin obtained from egg yolk. A substance that stabilizes an emulsion is called an emulsifier, emulgent, or surfactant. Detergents are a common group of surfactants. In addition, various emulsifiers are used to prepare facial creams and skin lotions.
Characteristics
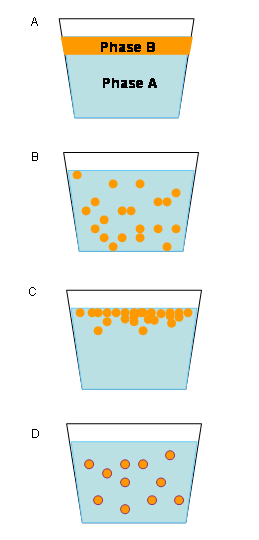
Emulsions tend to have a cloudy appearance, as a result of the scattering of light. As light passes through the emulsion, it is scattered by the many interfaces (boundaries) between the different phases (such as water and oil).
In addition, emulsions are unstable and do not form spontaneously. An example of an unstable emulsion is homemade salad dressing made by mixing oil and vinegar. The two phases separate quickly unless shaken repeatedly. The phases separate when smaller droplets recombine to form larger ones—a phenomenon called "coalescence." Fluid emulsions may also suffer from creaming—the migration of one of the substances to the top of the emulsion under the influence of buoyancy or centripetal force when a centrifuge is used.
Forming and stabilizing emulsions
To form an emulsion, the mixture of substances needs energy input through processes such as shaking, stirring, spraying, or homogenizing. As noted above, an emulsion can be stabilized by adding certain substances known as emulsifiers or surfactants (surface active agents). A good surfactant increases the kinetic stability[1] of the emulsion so greatly that the emulsion, once formed, does not change significantly over years of storage.
Types of emulsion instability
There are three types of emulsion instability:
- Breaking: The dispersed particles (droplets) coalesce and form a layer of liquid.
- Flocculation: The particles of the dispersed phase form clumps.
- Creaming: The particles of the dispersed phase concentrate near the surface of the mixture while staying separated.
Emulsifiers and their uses
Certain proteins, phospholipids, and low-molecular-weight substances can act as emulsifiers (or surfactants). Examples of food emulsifiers are egg yolk, in which the main emulsifying agent is the phospholipid lecithin, and mustard, in which a variety of chemicals in the mucilage surrounding the seed hull act as emulsifiers. Both mayonnaise and Hollandaise sauce are oil-in-water emulsions stabilized with egg yolk lecithin.
Detergents are another class of surfactants. They chemically interact with both oil and water, thus stabilizing the interfaces between oil and water. This principle is exploited in soap to remove grease for the purpose of cleaning. A wide variety of emulsifiers are used in pharmacy to prepare emulsions such as creams and lotions.
The production of a water-in-oil emulsion or an oil-in-water emulsion depends on the volume fraction of the two phases and the type of emulsifier. As a general rule, emulsifiers and emulsifying particles tend to promote dispersion of the phase in which they do not dissolve very well. This is called the "Bancroft rule." For example, certain proteins dissolve better in water than in oil, so they promote the dispersion of oil droplets throughout a continuous phase of water—that is, they promote the formation of oil-in-water emulsions.
See also
- Materials science
- Phase (matter)
Footnotes
- ↑ In this case, "kinetic stability" refers to stability of the movement of particles in the dispersed phase.
ReferencesISBN links support NWE through referral fees
- Sjoblom, Johan (ed.). Emulsions and Emulsion Stability (second edition). Surfactant Science Series Vol. 61, CRC, 2005. ISBN 0824726952.
- Sjoblom, Johan (ed.). Encyclopedic Handbook of Emulsion Technology, CRC, 2001. ISBN 0824704541.
- Becher, Paul. Emulsions: Theory and Practice 3rd ed. American Chemical Society, 2001. ISBN 0841234965.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.