Allotropy
If a chemical element can exist in two or more different forms, the forms are known as allotropes of the element, and this type of behavior is called allotropy. In each different allotrope, the element's atoms are bonded together in a different manner.
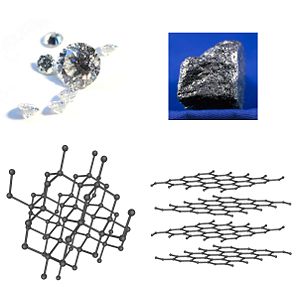
For example, the element carbon has two common allotropes: Diamond, in which the carbon atoms are bonded together in a tetrahedral lattice arrangement, and graphite, in which the carbon atoms are bonded together in sheets of a hexagonal lattice.
Note that allotropy refers only to different forms of an element within the same phase or state of matter (i.e. different solid, liquid, or gaseous forms). The changes of state between solid, liquid, and gas in themselves are not considered allotropy.
For some elements, allotropes can persist in different phasesâfor example, the two allotropes of oxygen (dioxygen and ozone), can both exist in the solid, liquid and gaseous states. Conversely, some elements do not maintain distinct allotropes in different phasesâfor example phosphorus has numerous solid allotropes, which all revert to the same P4 form when melted to the liquid state.
Etymology
The concept of allotropy was originally devised by the Swedish scientist Baron Jons Jakob Berzelius (1779-1848). The term allotropy is derived from the Greek words allos, meaning "other," and tropos, meaning "manner."
Differences in Properties of an Element's Allotropes
Allotropes of the same element can typically exhibit quite different physical properties and chemical behaviors, even though they contain nothing but atoms of that element. They may have different colors, odors, hardnesses, electrical and thermal conductivities, etc.
The change between different allotropic forms of an element is often triggered by pressure and temperature, and many allotropes are only stable in the correct conditions. For instance, iron only changes from ferrite to austenite above 1,333°F (723°C), and tin undergoes a process known as tin pest at 56°F (13.2°C) and below.
Examples of Allotropes
Typically, elements capable of variable coordination numbers and/or oxidation states tend to exhibit greater numbers of allotropic forms. Another contributing factor is the ability of an element to catenate. Allotropes are typically more noticeable in non-metals and metalloids.
Examples of allotropes include:
- diamondâan extremely hard, transparent crystal with the carbon atoms arranged in a tetrahedral lattice. A poor conductor.
- graphiteâa soft, black, flaky solid. A moderate electrical conductor. The C atoms are bonded in flat hexagonal lattices, which are then layered in sheets.
- fullereneâ(including the "buckyball," C60)
- Red Phosphorusâpolymeric solid
- White Phosphorusâcrystalline solid
- Black Phosphorusâsemiconductor, analogous to graphite
- dioxygen, O2âcolorless
- ozone, O3âblue
- tetraoxygen, O4âred
- Plastic (amorphous) sulfurâpolymeric solid
- Rhombic sulfurâlarge crystals composed of S8 molecules
- Monoclinic sulfurâfine needle-like crystals
- Molecular sulfurâtends to form ring molecules such as S7 and S12
Plutonium has six distinct solid allotropes under ânormalâ pressures. Their densities vary within a ratio of some 4:3, which vastly complicates all kinds of work with the metal (particularly casting, machining, and storage). A seventh plutonium allotrope exists at very high pressures, which adds further difficulties in exotic applications.
See also
ReferencesISBN links support NWE through referral fees
- Chang, Raymond. 2006. Chemistry. 9th ed. New York: McGraw-Hill Science/Engineering/Math. ISBN 0073221031 and ISBN 978-0073221038.
- Cotton, F. Albert, Geoffrey Wilkinson, Carlos A. Murillo, and Manfred Bochmann. 1999. Advanced Inorganic Chemistry. 6th ed. New York: Wiley. ISBN 0471199575.
- Greenwood, N.N., and A. Earnshaw. 1998. Chemistry of the Elements. 2nd ed. Oxford, U.K.; Burlington, MA: Butterworth-Heinemann, Elsevier Science. ISBN 0750633654. Online version available here. Retrieved August 11, 2007.
External links
All links retrieved July 21, 2023.
- IUPAC. Allotropes.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.