Covalent bond
Covalent bonding is a form of chemical bonding characterized by the sharing of one or more pairs of electrons between two elements, producing a mutual attraction that holds the resultant molecule together. Atoms tend to share electrons in such a way that their outer electron shells are filled. Such bonds are always stronger than the intermolecular hydrogen bond and similar in strength to or stronger than the ionic bond. While covalent bonding most often occurs between atoms, metals can form covalent bonds with other covalent bonds themselves which can complicate matters somewhat.
Covalent bonding most frequently occurs between atoms with similar electronegativities. For this reason, non-metals tend to engage in covalent bonding more readily since metals have access to metallic bonding, where the easily-removed electrons are freer to roam about. For non-metals, liberating an electron is more difficult, so sharing is the only option when confronted with another species of similar electronegativity.
However, covalent bonding in metals and, particularly between metals and organic compounds is particularly important, especially in industrial catalysis and process chemistry, where many indispensible reactions depend on covalent bonding with metals.
History
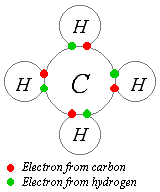
The idea of covalent bonding can be traced to Gilbert N. Lewis, who in 1916 described the sharing of electron pairs between atoms. He introduced the so called Lewis Notation or Electron Dot Notation in which valence electrons (those in the outer shell) are represented as dots around the atomic symbols. Pairs of electrons located between atoms represent covalent bonds. Multiple pairs represent multiple bonds, such as double and triple bonds. Some examples of Electron Dot Notation are shown in the following figure. An alternative form, in which bond-forming electron pairs are represented as solid lines, is shown in blue.
While the idea of shared electron pairs provides an effective qualitative picture of covalent bonding, quantum mechanics is needed to understand the nature of these bonds and predict the structures and properties of simple molecules. Heitler and London are credited with the first successful quantum mechanical explanation of a chemical bond, specifically that of molecular hydrogen, in 1927. Their work was based on the valence bond model, which assumes that a chemical bond is formed when there is good overlap between the atomic orbitals of participating atoms. These atomic orbitals are known to have specific angular relationships between each other, and thus the valence bond model can successfully predict the bond angles observed in simple molecules.
Bond order
Bond order is the scientific term used to describe the number of pairs of electrons shared between atoms forming a covalent bond. The most common type of covalent bond is the single bond, the sharing of only one pair of electrons between two individual atoms. All bonds with more than one shared pair are called multiple covalent bonds. The sharing of two pairs is called a double bond and the sharing of three pairs is called a triple bond. An example of a double bond is nitrous acid (between N and O), and an example of a triple bond is in hydrogen cyanide (between C and N).
A single bond usually consists of one sigma bond, a double bond of one sigma and one pi bond, and a triple bond of one sigma and two pi bonds.
Quadruple bonds, though rare, also exist. Both carbon and silicon can theoretically form these; however, the formed molecules are explosively unstable. Stable quadruple bonds are observed as transition metal-metal bonds, usually between two transition metal atoms in organometallic compounds. Molybdenum and Ruthenium are the elements most commonly observed with this bonding configuration. An example of a quadruple bond is also found in Di-tungsten tetra(hpp). Quintuple Bonds are found to exist in certain chromium dimers.
Sextuple bonds of order 6 have also been observed in transition metals in the gaseous phase at very low temperatures and are extremely rare.
Other more exotic bonds, such as three center bonds are known and defy the conventions of bond order. It is also important to note that bond order is an integer value only in the elementary sense and is often fractional in more advanced contexts.
Coordinate covalent bonds
A special case is called a dative covalent bond, also known as a coordinate covalent bond, which occurs when one atom gives both of the electrons in the bond.
Rigidity
Typically, two atoms can rotate about a single bond with relative ease. However, double and triple bonds are very difficult to rotate because they require pi orbital overlap. Pi orbital overlaps are parallel.
Resonance
Some structures can have more than one valid Lewis Dot Structure (for example, ozone, O3). In an LDS diagram of O3, the center atom will have a single bond with one atom and a double bond with the other. The LDS diagram cannot tell us which atom has the double bond; the first and second adjoining atoms have equal chances of having the double bond. These two possible structures are called resonance structures. In reality, the structure of ozone is a resonance hybrid between its two possible resonance structures. Instead of having one double bond and one single bond, there are actually two 1.5 bonds with approximately three electrons in each at all times.
A special resonance case is exhibited in aromatic rings of atoms (for example, benzene). Aromatic rings are composed of atoms arranged in a circle (held together by covalent bonds) that alternate between single and double bonds according to their LDS. In actuality, the electrons tend to be disambiguously and evenly spaced within the ring. Electron sharing in aromatic structures is often represented with a ring inside the circle of atoms.
Current theory
Today the valence bond model has been supplemented with the molecular orbital model. In this model, as atoms are brought together, the atomic orbitals interact to form hybrid molecular orbitals. These molecular orbitals are a cross between the original atomic orbitals and generally extend between the two bonding atoms.
Using quantum mechanics it is possible to calculate the electronic structure, energy levels, bond angles, bond distances, dipole moments, and frequency spectra of simple molecules with a high degree of accuracy. Currently, bond distances and angles can be calculated as accurately as they can be measured (distances to a few pm and bond angles to a few degrees). For the case small molecules, energy calculations are sufficiently accurate to be useful for determining thermodynamic heats of formation and kinetic activation energy barriers.
See also
- chemical bond
- ionic bond
- linear combination of atomic orbitals
- metallic bond
External links
ar:رابطة تساهمية bg:Ковалентна химична връзка ca:Enllaç covalent da:Kovalent de:Atombindung et:Kovalentne side es:Enlace covalente fa:پیوند کووالانسی fr:Liaison covalente he:קשר קוולנטי nl:Covalente binding ja:共有結合 nn:Kovalent binding pl:Wiązanie kowalencyjne pt:Ligação covalente fi:Kovalenttinen sidos sv:Kovalent bindning zh:共价键
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.