Difference between revisions of "Nitrogen fixation" - New World Encyclopedia
Rick Swarts (talk | contribs) (Added article and credit tags) |
|||
| Line 1: | Line 1: | ||
| + | {{Contracted}} | ||
'''Nitrogen fixation''' is the process by which [[nitrogen]] is taken from its relatively inert molecular form (N<sub>2</sub>) in the [[Earth's atmosphere|atmosphere]] and converted into nitrogen compounds useful for other chemical processes (such as, notably, [[ammonia]], [[nitrate]] and [[nitrogen dioxide]]). | '''Nitrogen fixation''' is the process by which [[nitrogen]] is taken from its relatively inert molecular form (N<sub>2</sub>) in the [[Earth's atmosphere|atmosphere]] and converted into nitrogen compounds useful for other chemical processes (such as, notably, [[ammonia]], [[nitrate]] and [[nitrogen dioxide]]). | ||
Revision as of 22:29, 10 May 2006
Nitrogen fixation is the process by which nitrogen is taken from its relatively inert molecular form (N2) in the atmosphere and converted into nitrogen compounds useful for other chemical processes (such as, notably, ammonia, nitrate and nitrogen dioxide).
Nitrogen fixation is performed naturally by a number of different prokaryotes, including bacteria, and actinobacteria certain types of anaerobic bacteria. Many higher plants, and some animals (termites), have formed associations with these microorganisms.
Biological Nitrogen Fixation
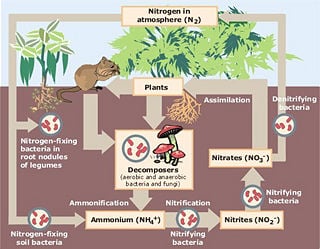
Biological Nitrogen Fixation (BNF) occurs when atmospheric nitrogen is converted to ammonia by a bacterial enzyme called nitrogenase. Microorganisms that fix nitrogen are called diazotrophs. The formula for BNF is:
- N2 + 8H+ + 8e- + 16 ATP → 2NH3 + H2 + 16ADP + 16 Pi
Although ammonia (NH3) is the direct product of this reaction, it is quickly ionized to ammonium (NH4+). In free-living diazotrophs, the nitrogenase-generated ammonium is assimilated into glutamate through the glutamine synthetase/glutamate synthase pathway. Biological nitrogen fixation was discovered by the Dutch microbiologist Martinus Beijerinck.
Leguminous nitrogen-fixing plants
The best-known are legumes (such as clover, beans, alfalfa and peanuts,) which contain symbiotic bacteria called rhizobia within nodules in their root systems, producing nitrogen compounds that help the plant to grow and compete with other plants. When the plant dies, the nitrogen helps to fertilize the soil. The great majority of legumes have this association, but a few genera (e.g., Styphnolobium) do not.
Non-leguminous nitrogen fixing plants
Plants from many other families have similar associations, including:
- Lobaria lichen and some other lichens
- Mosquito fern (Azolla species)
- Cycads
- Gunnera
- Alder (Alnus species)
- Ceanothus (Ceanothus species)
- Wax myrtle (Myrica species)
- Mountain-mahogany (Cercocarpus species)
- Bitterbrush (Purshia tridentata)
- Buffalo berry (Shepherdia argentea)
- Ironwood (Casuarina species), Sheoak (Allocasuarina species), and other genera in the Casuarinaceae
Chemical nitrogen fixation
Nitrogen can also be artificially fixed for use in fertilizer, explosives, or in other products. The most popular method is by the Haber process. Artificial fertilizer production has achieved such scale that it is now the largest source of fixed nitrogen in the Earth's ecosystem.
See also
- Denitrification
- George Washington Carver
- Nitrification
- Nitrogen cycle
- Nitrogen deficiency
External links
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.