Difference between revisions of "Dynamite" - New World Encyclopedia
(adding image of nitroglycerin) |
|||
| Line 1: | Line 1: | ||
| − | {{Approved}}{{Submitted}}{{Contracted}} | + | {{Approved}}{{Submitted}}{{Images OK}}{{Contracted}} |
| − | |||
'''Dynamite''' is the first safely manageable chemical explosive stronger than [[black powder]]*. It is based on the explosive potential of [[nitroglycerin]]*, with [[diatomaceous earth]]* (Kieselguhr) as an [[adsorbent]]*. Dynamite is considered a "high explosive", which means it [[detonation|detonates]]* rather than [[deflagration|deflagrates]]*. It was invented by [[Sweden|Swedish]] chemist and engineer [[Alfred Nobel]] in 1866, in Krümmel ([[Hamburg]]*, [[Germany]]), and [[patent|patented]]* in 1867. | '''Dynamite''' is the first safely manageable chemical explosive stronger than [[black powder]]*. It is based on the explosive potential of [[nitroglycerin]]*, with [[diatomaceous earth]]* (Kieselguhr) as an [[adsorbent]]*. Dynamite is considered a "high explosive", which means it [[detonation|detonates]]* rather than [[deflagration|deflagrates]]*. It was invented by [[Sweden|Swedish]] chemist and engineer [[Alfred Nobel]] in 1866, in Krümmel ([[Hamburg]]*, [[Germany]]), and [[patent|patented]]* in 1867. | ||
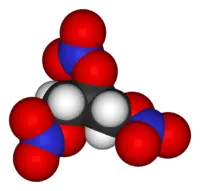
| − | [[Image:Nitroglycerin-3D-vdW.png|thumb|200px|3D model of nitroglycerin, the explosive component of dynamite.]] | + | [[Image:Nitroglycerin-3D-vdW.png|thumb|200px|3D model of a molecule of nitroglycerin, the explosive component of dynamite.]] |
==Etymology and history== | ==Etymology and history== | ||
| Line 18: | Line 17: | ||
== Chemical composition and properties == | == Chemical composition and properties == | ||
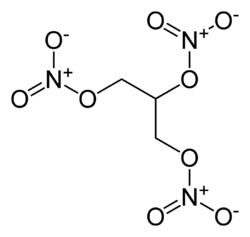
| + | [[Image:Nitroglycerin-2D-skeletal.png|thumb|250px|2D diagram of a molecule of nitroglycerin.]] | ||
Dynamite consists of three parts nitroglycerin, one part diatomaceous earth and a small admixture of [[sodium carbonate]]*. This mixture is formed into short sticks and wrapped in paper. Each stick is often 20 centimeters (cm) (roughly eight [[inch]]*es) long and 2.5 cm (one inch) in diameter, but other sizes also exist. | Dynamite consists of three parts nitroglycerin, one part diatomaceous earth and a small admixture of [[sodium carbonate]]*. This mixture is formed into short sticks and wrapped in paper. Each stick is often 20 centimeters (cm) (roughly eight [[inch]]*es) long and 2.5 cm (one inch) in diameter, but other sizes also exist. | ||
| Line 23: | Line 23: | ||
Nitroglycerin by itself is a very strong explosive. In its pure form, it is shock-sensitive, that is, physical shock can cause it to explode. It degrades over time to even more unstable forms. Consequently, it is highly dangerous to transport or use in its pure form. When absorbed into diatomaceous earth, however, nitroglycerin is less shock-sensitive. | Nitroglycerin by itself is a very strong explosive. In its pure form, it is shock-sensitive, that is, physical shock can cause it to explode. It degrades over time to even more unstable forms. Consequently, it is highly dangerous to transport or use in its pure form. When absorbed into diatomaceous earth, however, nitroglycerin is less shock-sensitive. | ||
| − | Over time, the dynamite stick will "weep" or "sweat" its | + | Over time, the dynamite stick will "weep" or "sweat" its nitroglycerin, which can then pool in the bottom of the box or storage area, and crystals form on the outside of the stick. This creates a very dangerous situation. Although the possibility of explosion without a [[blasting cap|cap]]* is minimal, old dynamite should not be handled. Qualified assistance should be sought immediately. |
A priming device is used to initiate the explosion of dynamite. A compartment within the shell contains explosive material that is more sensitive than the dynamite to be detonated. Also included in primer units are passageways that are open at both ends of the shell for receiving and housing an electric blasting cap. | A priming device is used to initiate the explosion of dynamite. A compartment within the shell contains explosive material that is more sensitive than the dynamite to be detonated. Also included in primer units are passageways that are open at both ends of the shell for receiving and housing an electric blasting cap. | ||
Revision as of 18:06, 9 January 2007
Dynamite is the first safely manageable chemical explosive stronger than black powder. It is based on the explosive potential of nitroglycerin, with diatomaceous earth (Kieselguhr) as an adsorbent. Dynamite is considered a "high explosive", which means it detonates rather than deflagrates. It was invented by Swedish chemist and engineer Alfred Nobel in 1866, in Krümmel (Hamburg, Germany), and patented in 1867.
Etymology and history
The word dynamite comes from the Greek word δυναμις (dunamis), meaning power, and the Greek suffix -ιτης (-itēs).
Nobel patented his invention in October 1867. He originally sold dynamite as "Nobel's Blasting Powder". After its introduction, dynamite rapidly gained popularity as a safe alternative to gunpowder and nitroglycerin. Nobel tightly controlled the patent and unlicensed duplicators were quickly shut down. Although a few U.S. businessmen got around the patent by using a slightly different formula, dynamite brought Nobel great wealth, which he used to found the Nobel Prize. After realizing dynamite's potential military uses, Nobel founded the prize as a way to promote peace and science for the benefit of mankind.
For several decades from the 1940s, the biggest producer of dynamite in the world was the Republic of South Africa, where De Beers established a factory in 1902 at Somerset West. The explosives factory was later operated by AECI (African Explosives and Chemical Industries). The demand for the product came mainly from the country's vast gold mines, centered on the Witwatersrand. The factory at Somerset West was in operation in 1903 and by 1907 was already producing 340,000 cases (weighing 50 pounds each) annually. A rival factory at Modderfontein was producing another 200,000 cases a year.[1]
One of the drawbacks of dynamite was that it was dangerous to manufacture. There were two massive explosions at the Somerset West plant in the 1960s, and some workers died. Yet, loss of life was limited by the modular design of the factory and earth works and plantations of trees that directed the blasts upwards. After 1985, production of dynamite at the factory was phased out.[2]
In the United States, dynamite was manufactured by the DuPont corporation well into the 1990s. It was eventually eclipsed by "water gel" explosives, which are safer to handle. [3]
Chemical composition and properties
Dynamite consists of three parts nitroglycerin, one part diatomaceous earth and a small admixture of sodium carbonate. This mixture is formed into short sticks and wrapped in paper. Each stick is often 20 centimeters (cm) (roughly eight inches) long and 2.5 cm (one inch) in diameter, but other sizes also exist.
Nitroglycerin by itself is a very strong explosive. In its pure form, it is shock-sensitive, that is, physical shock can cause it to explode. It degrades over time to even more unstable forms. Consequently, it is highly dangerous to transport or use in its pure form. When absorbed into diatomaceous earth, however, nitroglycerin is less shock-sensitive.
Over time, the dynamite stick will "weep" or "sweat" its nitroglycerin, which can then pool in the bottom of the box or storage area, and crystals form on the outside of the stick. This creates a very dangerous situation. Although the possibility of explosion without a cap is minimal, old dynamite should not be handled. Qualified assistance should be sought immediately.
A priming device is used to initiate the explosion of dynamite. A compartment within the shell contains explosive material that is more sensitive than the dynamite to be detonated. Also included in primer units are passageways that are open at both ends of the shell for receiving and housing an electric blasting cap.
Uses
The chief uses of dynamite used to be in construction, mining and demolition. However, newer explosives and techniques have replaced dynamite in many applications. Dynamite is still used, mainly as bottom charge or in underwater blasting. Dynamite has been used in armed conflicts, mainly to destroy bridges and other ways of travel, to slow the advance of supplies or enemy troops.
Dynamite in popular culture
The familiar thin reddish cylinder, equipped with a fuse or blasting cap, is a stock movie prop. In comedies and cartoons, dynamite commonly explodes with the only effect being a blackened face and wild hair. In dramas, the impending explosion of lit dynamite parcels provides movie tension. In action films, dynamite is often used as a weapon. In addition, dynamite can be found in many cartoon-style computer games and is usually very powerful in contrast to other weapons in a particular game.
Dynamite trivia
- In the computer game series "Worms", dynamite is used as a weapon and takes on the form of the classic thin reddish cylinder with a short fuse.
- In the television series Lost, episode "Exodus Part II", Jack, Kate, and Locke go into the Black Rock and find a crate containing dynamite sticks that had "sweated" (leaked) a great deal of nitroglycerin. While attempting to handle the explosives, one person is killed in an explosion.
- In a 1963 episode of The Andy Griffith Show titled "The Loaded Goat", Jimmy the goat devours a caseload of dynamite before roaming the streets of Mayberry.
- "A Fistful of Dynamite" is a 1971 film by Sergio Leone in which James Coburn plays an ex-IRA explosives expert.
- In the 1975 Disney comedy movie The Apple Dumpling Gang, Tim Conway and Don Knotts use sweating dynamite to conduct a bank robbery.
- In the 1977 film Sorcerer, directed by William Friedkin, the main plot focused on four fugitives working for a South American oil camp after they had escaped their criminal pasts. They volunteered to transport four cases of old dynamite that had "sweated" out nitroglycerin to put out an oil-well fire, they are forced to travel through 220 miles of jungle, mountain, and desert terrain. This film was a remake of The Wages of Fear (Le Salaire de la peur) by Henri-Georges Clouzot, in which the drivers transported nitroglycerin in cans.
- On the '70s U.S. television program about an impoverished African-American family living in Chicago, "Good Times" had J.J. (played by Jimmy Walker) continuously shouting "Dyn-o-mite."
- Dynamite was a children's magazine published in the 1970s.
- The popular TV show "Myth Busters" uses dynamite in several of their episodes.
- In 2005, the British band Jamiroquai released their sixth album named "Dynamite".
- The movie Sahara shows the use of dynamite to create a smokescreen and unearth a civil war battleship in the sand.
Dynamite as a metaphor
The term dynamite may also be used in a metaphorical sense. For instance, one may describe a controversial (and possibly scandalous) public issue as political dynamite, or an exciting game as a dynamite game.
See also
Patents
- U.S. Patent 0078317 (PDF), Improved explosive compound
- U.S. Patent 3931763 (PDF), Explosive priming device
ReferencesISBN links support NWE through referral fees
- Akhavan, J. (2004). The Chemistry of Explosives (2nd edition). Cambridge, UK: The Royal Society of Chemistry. ISBN 0854046402.
- Cooper, Paul W., and Kurowski, Stanley, R. (1996). Introduction to the Technology of Explosives. New York, NY: Wiley-VCH. ISBN 047118635X.
- Cooper, Paul W. (1996). Explosives Engineering. New York, NY: Wiley-VCH. ISBN 0471186368.
- Meyer, Rudolf; Kohler, Josef; and Homburg, Axel (2002). Explosives (5th revised edition). New York, NY: Wiley-VCH. ISBN 3527302670.
External links
- Oregon State Police - Arson and Explosives Section (Handling instructions and photos)
- Big bang
- Detonator cables
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.