Macular degeneration
| [[Image:{{{Image}}}|190px|center|]] | |
|---|---|
| ICD-10 | H35.3 |
| ICD-O: | {{{ICDO}}} |
| ICD-9 | 362.5 |
| OMIM | {{{OMIM}}} |
| MedlinePlus | {{{MedlinePlus}}} |
| eMedicine | {{{eMedicineSubj}}}/{{{eMedicineTopic}}} |
| DiseasesDB | {{{DiseasesDB}}} |
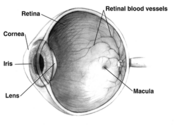
Macular degeneration is a medical condition predominantly found in elderly adults in which the center of the inner lining of the eye, known as the macula area of the retina, suffers thinning, atrophy, and in some cases bleeding. This can result in loss of central vision, which entails inability to see fine details, to read, or to recognize faces. According to the American Academy of Ophthalmology, it is the leading cause of central vision loss (blindness) and in the United States today for those over the age of fifty years. Although some macular dystrophies that affect younger individuals are sometimes referred to as macular degeneration, the term generally refers to age-related macular degeneration (AMD or ARMD).
Age-related macular degeneration begins with characteristic yellow deposits in the macula (central area of the retina) called drusen. Most people with these early changes have good vision. People with drusen can go on to develop advanced AMD. The risk is considerably higher when the drusen are large and numerous and associated with disturbance in the pigmented cell layer under the macula. Recent research suggests that large and soft drusen are related to elevated cholesterol deposits and may respond to cholesterol lowering agents or the Rheo Procedure.
Advanced AMD, which is responsible for profound vision loss, has two forms: dry and wet. Central geographic atrophy, the dry form of advanced AMD, results from atrophy to the retinal pigment epithelial layer below the retina, which causes vision loss through loss of photoreceptors (rods and cones) in the central part of the eye. While no treatment is available for this condition, vitamin supplements with high doses of antioxidants, Lutein and Zeaxanthin, have been demonstrated by the National Eye Institute and others to slow the progression of dry macular degeneration and in some patients, improve visual acuity.
Neovascular or exudative AMD, the wet form of advanced AMD, causes vision loss due to abnormal blood vessel growth in the choriocapillaries, through Bruch's membrane, ultimately leading to blood and protein leakage below the macula. Bleeding, leaking, and scarring from these blood vessels eventually cause irreversible damage to the photoreceptors and rapid vision loss if left untreated.
Until recently, no effective treatments were known for wet macular degeneration. However, new drugs, called anti-VEGF (anti-Vascular Endothelial Growth Factor) agents, when injected directly into the vitreous humor of the eye using a small, painless needle, can cause contraction of the abnormal blood vessels and improvement of vision. The injections frequently have to be repeated on a monthly or bi-monthly basis. Examples of these agents include Lucentis, Avastin and Macugen. Only Lucentis and Macugen are FDA approved as of April 2007, and only Lucentis and Avastin appear to be able to improve vision, but the improvements are slight and do not restore full vision.
Risk factors
- Aging: Approximately 10% of patients 66 to 74 years of age will have findings of macular degeneration. The prevalence increases to 30% in patients 75 to 85 years of age.[citation needed]
- Smoking: The only environmental exposure clearly associated with macular degeneration is tobacco smoking.[1] Exposure to cigarette smoke more than doubles the risk of macular degeneration.[citation needed]
- Family history: The lifetime risk of developing late-stage macular degeneration is 50% for people who have a relative with macular degeneration vs. 12% for people who do not have relatives with macular degeneration, i.e. a four fold higher risk.[citation needed]
- Macular degeneration gene: The genes for the complement system proteins factor H (CFH) and factor B (CFB) have been determined to be strongly associated with a person's risk for developing macular degeneration. CFH is involved in inhibiting the inflammatory response mediated via C3b (and the Alternative Pathway of complement) both by acting as a cofactor for cleavage of C3b to its inactive form, C3bi, and by weakening the active complex that forms between C3b and factor B. C-reactive protein and polyanionic surface markers such as glycosaminoglycans normally enhance the ability of factor H to inhibit complement . But the mutation in CFH(Tyr402His) reduces the affinity of CFH for CRP and probably also alters the ability of factor H to recognise specific glycosaminoglycans. This change results in reduced ability of CFH to regulate complement on critical surfaces such as the specialised membrane at the back of the eye and leads to increased inflammatory response within the macula. In two 2006 studies at Yale Department of Epidemiology and Public Health and the Department of Ophthalmology and Visual Sciences, Moran Eye Center at the University of Utah School of Medicine, another gene that has implications for the disease, called HTRA1 (encoding a secreted serine protease), was identified. [2][3]
- Hypertension: Also known as high blood pressure.
- Cardiovascular status - high cholesterol, obesity.
- High fat intake is associated with an increased risk of macular degeneration in both women and men. Fat provides about 42% of the food energy in the average American diet. A diet that derives closer to 20-25% of total food energy from fat is probably healthier. Reducing fat intake to this level means cutting down greatly on consumption of red meats and dairy products such as milk, cheese, and butter. Eating more cold-water fish[4] (at least twice weekly), rather than red meats, and eating any type of nuts may help macular degeneration patients.[5]
- Oxidative stress: It has been proposed that age related accumulation of low molecular weight, phototoxic, pro-oxidant melanin oligomers within lysosomes in the retinal pigment epithelium may be partly responsible for decreasing the digestive rate of photoreceptor outer rod segments (POS) by the RPE. A decrease in the digestive rate of POS has been shown to be associated with lipofuscin formation - a classic sign associated with macular degeneration.[6]
- Race Macular degeneration is more likely to be found in whites than in blacks.[7][8]
- Exposure to sunlight especially blue light. There is conflicting evidence as to whether exposure to sunlight contributes to the development of macular degeneration. A recent study in the British Journal of Ophthalmology on 446 subjects found that it does not.[9] High energy visible light (HEV) has been implicated as a cause of age-related macular degeneration.[10][11]
Signs
- Drusen
- Pigmentary alterations
- Exudative changes: hemorrhages in the eye, hard exudates, subretinal/sub-RPE/intraretinal fluid
- Atrophy: incipient and geographic
- Visual acuity drastically decreasing (two levels or more) ex: 20/20 to 20/80.
Symptoms
- Blurred vision: Those with nonexudative macular degeneration may by asymptomatic or notice a gradual loss of central vision, whereas those with exudative macular degeneration often notice a rapid onset of vision loss.
- Central scotomas (shadows or missing areas of vision)
- Distorted vision (i.e. metamorphopsia) - A grid of straight lines appears wavy and parts of the grid may appear blank. Patients often first notice this when looking at mini-blinds in their home.
- Trouble discerning colors; specifically dark ones from dark ones and light ones from light ones.
- Slow recovery of visual function after exposure to bright light
The Amsler Grid Test is one of the simplest and most effective methods for patients to monitor the health of the macula. The Amsler Grid is essentially a pattern of intersecting lines (identical to graph paper) with a black dot in the middle. The central black dot is used for fixation (a place for the eye to stare at). With normal vision, all lines surrounding the black dot will look straight and evenly spaced with no missing or odd looking areas when fixating on the grid's central black dot. When there is disease affecting the macula, as in macular degeneration, the lines can look bent, distorted and/or missing.
'Vision loss' or 'blindness' in macular degeneration refers to the loss of 'central vision' only. The peripheral vision is preserved. Blindness in macular degeneration does not mean 'inability to see light' and even with far advanced macular degeneration, the peripheral retina allows for useful vision.
The loss of central vision profoundly affects visual functioning. It is not possible, for example, to read without central vision. Pictures which attempt to depict the central visual loss of macular degeneration with a black spot do not really do justice to the devastating nature of the visual loss. This can be demonstrated by printing letters 6 inches high on a piece of paper and attempting to identify them while looking straight ahead and holding the paper slightly to the side. Most people find this surprisingly difficult to do.
Similar symptoms with a very different etiology and different treatment can be caused by Epiretinal membrane or macular puckeror leaking blood vessels in the eye..
Diagnosis
Fluorescein angiography allows for the identification and localization of abnormal vascular processes. Optical coherence tomography is now used by most ophthalmologists in the diagnosis and the followup evaluation of the response to treatment by using either Avastin or Lucentis which are injected into the vitreous of the eye at various intervals.
Treatment
Most of the treatments that are available now and and currently being studied are aimed at stopping the neovascular (or wet) form of AMD.
In June 2006, the drug ranibizumab (Lucentis) has been approved by the FDA for use in the treatment of AMD.[12] Ranibizumab has been shown to halt the progression of the disease in most patients receiving the treatment. Unlike previous treatments, a significant majority (70%) receiving ranibizumab had an improvement in vision of at least 1 letter. Up to 40% of patients had a significant vision increase of 3 lines or more. In addition, up to 50% had a vision of 20/40 or better after 12 months of treatment. This is significant as 20/40 is commonly seen as the vision at which a person can still drive a car. Ranibizumab was the first therapy to show a statistically significant improvement in patient reported outcomes.[13][14] Ranibizumab is given as an injection into the eye. The initial studies required an injection every 4 weeks for 2 years.
Bevacizumab (Avastin), a drug approved for use in colon cancer, has been used by ophthalmologists in the treatment of wet macular degeneration. Bevacizumab and ranibizumab were developed for the same monoclonal antibody parent. However, ranibizumab has been affinity matured 140x and is a much smaller molecule than bevazicumab. Being smaller allows ranibizumab to penetrate all layers of the retina and also to clear faster systemically from the eye. Doubts about whether bevacizumab can penetrate the layers of the retina led to the development of ranibizumab. There are also concerns about the safety of bevacizumab as it known to have significant systemic effects. Before Lucentis was available, bevacizumab was widely used by ophthalmologists who treat macular degeneration. Some of their experiences with large numbers of patients with relatively short follow-up times were recently published. No randomized controlled clinical trial with systematic safety data collection has been performed to validate its efficacy and safety with same certainty as ranibizumab. Bevacizumab, when administered at the usual cancer treatment doses, has been shown to cause systemic adverse effects. The most common adverse effect was hypertension. There is a continued interest as the bevacizumab for use in the eye can be obtained for about 30-50 dollars per dose, compared to 2,000 dollars per dose for ranibizumab. One concern is that bevacizumab is aliquotted out by compounding pharmacies from a single use vial of bevacizumab. This may lead to degradation and impurities within the product. Following the recommended protocol for ranibizumab costs about $50,000 per eye over two years. The National Eye Institute is planning a head-to-head ranibizumab vs. bevacizumab, randomized, controlled clinical trial for treatment of macular degeneration. Currently more than 50% of retinal specialists use bevacizumab as the first line drug (ACRS Practice Patterns Survey).
Pegaptanib (Macugen) was approved in 2004 for treatment of neovascular AMD. It targets certain forms of VEGF molecules and is injected directly into the eye like ranibizumab or bevacizumab. Although this was shown to decrease the risk of vision loss significantly compared to no treatment, it is felt to be relatively ineffective compared to the newer treatments.
Photodynamic therapy (PDT) with verteporfin (Visudyne) had been the treatment of choice for neovascular AMD until recently. This was the first treatment shown to decrease the chance of severe vision loss in 2 years in patients with neovascular AMD without first causing immediate vision loss at the time of the treatment. A photosenstive dye with affinity for the abnormal blood vessels are first injected through the veins. A low-energy activating laser is then directed toward the abnormal blood vessels, causing selective damage to those blood vessels. This has also fallen out of favor as newer, more effective treatments became available.
Direct laser treatment for neovascular AMD was shown to decrease the chance of profound vision loss at 2 years in patients with neovascular AMD but it is seldom used as the treatment itself causes significant vision loss immediately. Infrequently, abnormal blood vessels outside of the center part of the macula are detected. Direct laser treatment can be an effective way to treat these patients with acceptable morbidity.
Other drugs that are currently under investigation include: anecortave (Retaane), squalamine (Evizon), VEGF TRAP-EYE (made by Regeneron), and siRNA. Second generation antisense oligonucleotides iCo-007 targeting the Raf-1 kinase are also under investigation as a target for broad inhibition of multiple pro-angiogenic signals. Radiation therapy (brachytherapy) and rheopheresis are also being evaluated for wet macular degeneration.[15]
None of the drugs or laser treatment can restore vision to patients that have already suffered permanent damage to the photoreceptors or retinal pigmented epithelial cells due to advanced forms of AMD. Stem cells are currently being studied as a potential solution to this problem.
OT-551 eyedrop is currently being evaluated in an National Eye Institute-sponsored trial as a treatment for the dry form of AMD (the drug is already under investigation as a treatment for cataracts).
Prevention
The Age-Related Eye Disease Study showed that a combination of high-dose beta-carotene, vitamin C, vitamin E, and zinc can reduce the risk of developing advanced AMD by about 25 percent in those patients who have earlier but significant forms of the disease. This is the only proven intervention to decrease the risk of advanced AMD at this time. A follow up study, Age-Related Eye Disease Study 2 to study the potential benefits of lutein, zeaxanthine, and fish oil, is currently underway.
Anecortave acetate, (Retanne), is an anti-angiogenic drug that is given as an injection behind the eye (avoiding an injection directly into the eye) that is currently being studied as a potential way of reducing the risk of neovascular (or wet) AMD in high-risk patients.
Recent studies suggest that statins, a family of drugs used for reducing cholesterol levels, may be effective in prevention of AMD, and in slowing its progression.[16]
Juvenile macular degeneration
Juvenile macular degeneration is not a term in standard usage at this time. The preferred term for conditions that affect the macula in younger individuals related to genetics is macular dystrophy. Examples of these include:
- Best's disease
- Doyne's honeycomb retinal dystrophy
- Sorsby's disease
- Stargardt's disease
Impact
Macular degeneration, in its advanced forms, can result in legal blindness, resulting in a loss of driving privileges and an inability to read all but very large type. Perhaps the most grievous loss is the inability to see faces clearly or at all.
Some of these losses can be offset by the use of adaptive devices. A closed-circuit television reader can make reading possible, and specialized screen-reading computer software, e.g., JAWS for Windows, can give the blind person access to word processing, spreadsheet, financial, and e-mail access.
ReferencesISBN links support NWE through referral fees
- ↑ http://news.bbc.co.uk/2/hi/health/4217010.stm
- ↑ Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, Dewan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K. "A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration." Science. 2006 Nov 10;314(5801):992-3. PMID 17053109.
- ↑ Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J. "A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration". Science. 2006 Nov 10;314(5801):989-92. PMID 17053108
- ↑ John Paul SanGiovanni, ScD; Emily Y. Chew, MD; Traci E. Clemons, PhD; Matthew D. Davis, MD; Frederick L. Ferris III, MD; Gary R. Gensler, MS; Natalie Kurinij, PhD; Anne S. Lindblad, PhD; Roy C. Milton, PhD; Johanna M. Seddon, MD; and Robert D. Sperduto, MD (May 5, 2007). The Relationship of Dietary Lipid Intake and Age-Related Macular Degeneration in a Case-Control Study. Archives of Ophthamology.
- ↑ Macular degeneration Types and Risk Factors
- ↑ "Melanin aggregation and polymerization: possible implications in age related macular degeneration." Ophthalmic Research, 2005; volume 37: pages 136-141.
- ↑ Age-Related Eye Disease Study Research Group. "Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3." Ophthalmology. 2000 Dec;107(12):2224-32. PMID 11097601.
- ↑ Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL 3rd; Age-Related Eye Disease Study Research Group. "Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19." Ophthalmology. 2005 Apr;112(4):533-9. PMID 15808240.
- ↑ Khan, JC and Shahid H, Thurlby DA, Bradley M, Clayton DG, Moore AT, Bird AC, Yates JR, Genetic Factors in AMD Study (Jan 2006). Age related macular degeneration and sun exposure, iris colour, and skin sensitivity to sunlight. The British Journal of Ophthalmology 90 (1): 29-32. PMID 16361662.
- ↑ Glazer-Hockstein, C and Dunaief JL (Jan 2006). Could blue light-blocking lenses decrease the risk of age-related macular degeneration?. Retina 26 (1): 1-4. PMID 16395131.
- ↑ Margrain, TH and Boulton M, Marshall J, Sliney DH (Sep 2004). Do blue light filters confer protection against age-related macular degeneration?. Progress in Retinal and Eye Research 23 (5): 523-31. PMID 15302349.
- ↑ United States Food and Drug Administration (2006-06-30). FDA Approves New Biologic Treatment for Wet Age-Related Macular Degeneration. Press release. Retrieved on 2006-10-23.
- ↑ Brown, DM and Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S; ANCHOR Study Group (Oct 5 2006). Ranibizumab versus verteporfin for neovascular age-related macular degeneration. New England Journal of Medicine 355 (14): 1432-44. PMID 17021319.
- ↑ Rosenfeld, PJ and Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY; MARINA Study Group (Oct 5 2006). Ranibizumab for neovascular age-related macular degeneration. New England Journal of Medicine 355 (14): 1419-31. PMID 17021318.
- ↑ http://www.agingeye.net/maculardegen/maculardegennewdevelopments.php
- ↑ http://bjo.bmjjournals.com/cgi/content/full/882/161
External links
- Macular Degeneration Support
- Results from the National Eye Institute's Age Related Eye Disease Study showed that high levels of antioxidants and zinc significantly reduce the risk of advanced age-related macular degeneration (AMD) and its associated vision loss.
- An open-access eBook on Macular Degeneration: Protect Your Sight - How to Save your Vision in the Epidemic of Age-Related Macular Degeneration by James C. Folk, MD and Mark E. Wilkinson, OD - Published March 2006
- Macular Degeneration Research
- Macular Degeneration 3000 word report A Patients Guide to AMD
- Rheo.com |The RHEO procedure is a unique type of therapeutic apheresis that removes large molecules like cholesterol, fibrinogen and certain immunoglobulins from the blood.
- Home of Ocular Surgery News
- BBC News — AMD from a European perspective
- Macular Degeneration Foundation
- Living with Macular Degeneration. NLM. Retrieved 2007-04-15. video
- VisionSimulations.com |What the world looks like to people with various diseases and conditions of the eye
- Why good food isn't enough - Powerful antioxidant vitamin supplements can halt the progression of dry macular degeneration of the eye and in some people even improve vision.
- ARMD Cook |Recipes using medical guidelines for the prevention and possible reversal of MD and ARMD
- Wellman Center for Photomedicine at Massachusetts General Hospital and Harvard Medical School information on the use of photomedicine as treatment
- Age Related Macular Degeneration amdcanada.com
- Scientists seek to restore sight. BBC News Scotland (2006-07-11). Retrieved 2006-07-13.
- BBC News, Friday, 16 March 2007; Zinc could be key to eye disease
- MyVisionTest A free modern Amsler Grid test for macular degeneration
- AMD Alliance International
| Pathology of the eye (primarily H00-H59) | |
|---|---|
| Eyelid, lacrimal system and orbit | Stye - Chalazion - Blepharitis - Entropion - Ectropion - Lagophthalmos - Blepharochalasis - Ptosis - Xanthelasma - Trichiasis - Dacryoadenitis - Epiphora - Exophthalmos - Enophthalmos |
| Conjunctiva | Conjunctivitis - Pterygium - Subconjunctival hemorrhage |
| Sclera and cornea | Scleritis - Keratitis - Corneal ulcer - Snow blindness - Thygeson's superficial punctate keratopathy - Fuchs' dystrophy - Keratoconus - Keratoconjunctivitis sicca - Arc eye - Keratoconjunctivitis - Corneal neovascularization - Kayser-Fleischer ring - Arcus senilis |
| Iris and ciliary body | Iritis - Uveitis - Iridocyclitis - Hyphema - Persistent pupillary membrane |
| Lens | Cataract - Aphakia |
| Choroid and retina | Retinal detachment - Retinoschisis - Hypertensive retinopathy - Diabetic retinopathy - Retinopathy - Retinopathy of prematurity - Macular degeneration - Retinitis pigmentosa - Macular edema - Epiretinal membrane - Macular pucker |
| Ocular muscles, binocular movement, accommodation and refraction | Strabismus - Ophthalmoparesis - Progressive external ophthalmoplegia - Esotropia - Exotropia - Refractive error - Hyperopia - Myopia - Astigmatism - Anisometropia - Presbyopia - Fourth nerve palsy - Sixth nerve palsy - Kearns-Sayre syndrome - Esophoria - Exophoria - Duane syndrome - Convergence insufficiency - Internuclear ophthalmoplegia - Aniseikonia |
| Visual disturbances and blindness | Amblyopia - Leber's congenital amaurosis - Subjective (Asthenopia, Hemeralopia, Photophobia, Scintillating scotoma) - Diplopia - Scotoma - Anopsia (Binasal hemianopsia, Bitemporal hemianopsia, Homonymous hemianopsia, Quadrantanopia) - Color blindness (Achromatopsia) - Nyctalopia - Blindness/Low vision |
| Commonly associated infectious diseases | Trachoma - Onchocerciasis |
| Other | Glaucoma - Floater - Leber's hereditary optic neuropathy - Red eye - Argyll Robertson pupil - Keratomycosis - Xerophthalmia - Aniridia |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.