Urea
| Urea | |
|---|---|
| |
| General | |
| Systematic name | Diaminomethanal |
| Other names | carbamide |
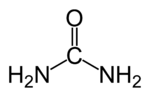
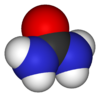
| Molecular formula | (NH2)2CO |
| SMILES | NC(=O)N |
| Molar mass | 60.07 g/mol |
| Appearance | white odourless solid |
| CAS number | [57-13-6] |
| Properties | |
| Density and phase | 1.33•103 kg/m3,[1] solid |
| Solubility in water | 108 g/100 ml (20 °C) 167 g/100 ml (40 °C) 251 g/100 ml (60 °C) 400 g/100 ml (80 °C) 733 g/100 ml (100 °C) |
| Melting point | 132.7 °C (406 K) decomposes |
| Boiling point | n.a. |
| Acidity (pKa) | 0.18 |
| Basicity (pKb) | 13.82 |
| Chiral rotation [α]D | Not chiral |
| Viscosity | ? cP at ? °C |
| Critical relative humidity | 81% (20 °C) 73% (30 °C) |
| Heat of solution in water | -57,8 cal/g (endothermic) |
| Nitrogen content | 46,6 %N |
| Structure | |
| Molecular shape | ? |
| Coordination geometry | trigonal planar |
| Crystal structure | tetragonal |
| Dipole moment | 4.56 p/D |
| Hazards | |
| MSDS | J.T. Baker |
| Main hazards | Toxic |
| Flash point | ? °C |
| R/S statement | R: ? S: ? |
| RTECS number | ? |
| NFPA 704 | estimated |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | ? |
| Other cations | ? |
| Related ? | biuret triuret thiourea |
| Related compounds | ? |
| Except where noted otherwise, data are given for materials in their standard state (at 26°C, 100 kPa) | |
Urea is an organic compound of carbon, nitrogen, oxygen, and hydrogen. Its chemical formula may be written as CO(NH2)2, CON2H4, or CN2H4O. It is also known as carbamide, carbamide resin, isourea, carbonyl diamide, and carbonyldiamine.[2]
Urea is found in mammalian and amphibian urine as well as in some fishes.[3] It was the first organic compound to be artificially synthesized from inorganic starting materials, thereby dealing a serious blow to the theory of vitalism.
Besides its physiological role, urea has many practical applications. For instance, it is a component of fertilizers and animal feed; a raw material for the production of certain plastics and adhesives; a flame-proofing agent; an ingredient in some hair conditioners, facial cleansers, and bath oils; an ingredient in various tooth whitening products; a substance that reduces nitrogen oxides in diesel-engine exhausts; an agent used in the dyeing and printing of textiles; a chemical for denaturing proteins in the laboratory; an agent used in the dyeing and printing of textiles; and an ingredient in products that promote rehydration of the skin. Commercial production of urea is on the order of 100,000,000 tons per year worldwide.
Discovery
Urea was discovered by Hilaire Rouelle in 1773. In 1828, in attempting to prepare ammonium cyanate, Friedrich Wöhler reacted potassium cyanate with ammonium sulfate. He inadvertently obtained urea, which thus became the first organic compound to be artificially synthesized from inorganic starting materials. This result dealt a severe blow to the concept of vitalism—the belief that chemicals that originated in living organisms could only be produced with the assistance of a "vital force" (present in living tissue) and could not be artificially synthesized.
Physiological roles
The individual atoms that make up a urea molecule come from carbon dioxide, water, aspartate and ammonia in a metabolic pathway known as the urea cycle, an anabolic process. This expenditure of energy is necessary because ammonia, a common metabolic waste product, is toxic and must be neutralized. Urea production occurs in the liver and is under the regulatory control of N-acetylglutamate.
Most organisms have to deal with the excretion of nitrogen waste originating from protein and amino acid catabolism. In aquatic organisms the most common form of nitrogen waste is ammonia, while land-dwelling organisms developed ways to convert the toxic ammonia to either urea or uric acid. Generally, birds and saurian reptiles excrete uric acid, while the remaining species, including mammals, excrete urea. Remarkably, tadpoles excrete ammonia, and shift to urea production during metamorphosis. In veterinary medicine, dalmatian breeds of dogs are different in that they excrete urea in the form of uric acid in the urine rather than in the urea form. This is due to a defect in one of the genes controlling expression of the conversion enzymes in the urea cycle.
The urea is formed in the livers of mammals in a cyclic pathway, from the break down of ammonia, (a metabolic waste), which was initially named the Krebs-Henseleit cycle after its discoverers, and later became known simply as the urea cycle. This cycle was partially deduced by Hans Adolf Krebs and Kurt Henseleit in 1932 and was clarified in the 1940s as the roles of citrulline and argininosuccinate as intermediates were understood.
In this cycle, amino groups donated by ammonia and L-aspartate are converted to urea, while L-ornithine, citrulline, L-arginino-succinate, and L-arginine act as intermediates.
Despite the generalization above, the pathway has been documented not only in mammals and amphibians, but in many other organisms as well, including birds, invertebrates, insects, plants, yeast, fungi, and even microorganisms.
Urea is essentially a waste product, although it is used by the body during times of volume reduction. It is dissolved in blood (in humans at a concentration of 2.5–7.5 mmol/liter) and excreted by the kidney in the urine. In addition, a small amount of urea is excreted (along with sodium chloride and water) in human sweat.
Production
Urea is produced commercially from synthetic ammonia and carbon dioxide. Urea can be produced as prills, granules, flakes, pellets, crystals and solutions.
More than 90 percent of world production is destined for use as a fertilizer. Urea has the highest nitrogen content of all solid nitrogenous fertilizers in common use (46.4 percent) It therefore has the lowest transportation costs per unit of nitrogen nutrient.
Urea is highly soluble in water and is therefore also very suitable for use in fertilizer solutions (in combination with ammonium nitrate: UAN), e.g. in 'foliar feed' fertilizers.
Solid urea is marketed as prills or granules. The advantage of prills is that in general they can be produced more cheaply than granules which, because of their narrower particle size distribution have an advantage over prills if applied mechanically to the soil. Properties such as impact strength, crushing strength and free-flowing behavior are particularly important in product handling, storage, and bulk transportation.
Commercial production
Urea is produced commercially from two raw materials: ammonia and carbon dioxide. Large quantities of carbon dioxide are produced during the manufacture of ammonia from coal or from hydrocarbons such as natural gas and petroleum derived raw materials. This allows direct synthesis of urea from these raw materials.
The production of urea from ammonia and carbon dioxide takes place in an equilibrium reaction, with incomplete conversion of the reactants. The various urea processes are characterized by the conditions under which urea formation takes place and the way in which unconverted reactants are further processed.
Unconverted reactants can be used for the manufacture of other products, for example ammonium nitrate or sulfate, or they can be recycled for complete conversion to urea in a total-recycle process.
Two principal reactions take place in the formation of urea from ammonia and carbon dioxide. The first reaction is exothermic (it gives off heat):
- 2NH3 + CO2 → H2N-COONH4 (ammonium carbamate)
The second reaction is endothermic (it absorbs heat):
- H2N-COONH4 → (NH2)2CO + H2O
The combination of the two reactions is exothermic.
Uses
Commercial uses
- As a component of fertilizer and animal feed, providing a relatively cheap source of fixed nitrogen to promote growth.
- As a raw material for the manufacture of plastics specifically, urea-formaldehyde resin.
- As a raw material for the manufacture of various glues (urea-formaldehyde or urea-melamine-formaldehyde). The latter is waterproof and is used for marine plywood.
- As an alternative to rock salt in the deicing of roadways and runways. It does not promote metal corrosion to the extent that salt does.
- As an additive ingredient in cigarettes, designed to enhance flavor.
- Sometimes used as a browning agent in factory-produced pretzels.
- As an ingredient in some hair conditioners, facial cleansers, bath oils and lotions.
- It is also used as a reactant in some ready-to-use cold compresses for first-aid use, due to the endothermic reaction it creates when mixed with water.
- Active ingredient for diesel-engine exhaust treatment AdBlue and some other SCR systems.
- Used, along with salts, as a cloud seeding agent to expedite the condensation of water in clouds, producing precipitation.
- The ability of urea to form clathrates (also called host-guest complexes, inclusion compounds, and adducts) was used in the past to separate paraffins.
- As a flame-proofing agent.
- As a clean burning fuel for motor vehicles and stationary engines.
- As a NOx-reducing reactant in combustion exhaust streams, especially diesel.
- As an ingredient in many tooth whitening products.
- Used in coal fired power plants to reduce emissions of nitrogen oxides.
- As an agent in the dyeing and printing of textiles, it provides solubility to the bath and retains some moisture, which is required for the dyeing or printing process.
Laboratory uses
- Urea is a powerful protein denaturant, as it disrupts the noncovalent bonds in proteins. This property can be exploited to increase the solubility of some proteins. For this application, it is used in concentrations up to 10 M.
- Urea is an ingredient in the synthesis of urea nitrate.
Medical uses
- Drug uses
Urea is used in topical dermatological products to promote rehydration of the skin. If covered by an occlusive dressing, 40 percent urea preparations may be used for nonsurgical debridement of nails.
- Some diagnostic uses
Isotopically labeled urea (carbon 14-radioactive, or carbon 13-stable isotope) is used in the Urea breath test, which helps detect the presence of certain bacteria (Helicobacter pylori) in the stomach and duodenum of humans. The test detects the characteristic enzyme urease, produced by H. pylori, by a reaction that produces ammonia from urea. This increases the pH (reduces acidity) of the stomach environment around the bacteria.
Bacterial species similar to H. pylori can be identified by the same test in various animals, such as apes, dogs, and cats (including big cats).
Related compounds
Ureas or carbamides are a class of chemical compounds sharing the same functional group RR'N-CO-NRR' based on a carbonyl group flanked by two organic amine residues. They can be accessed in the laboratory by reaction of phosgene with primary or secondary amines. Example of ureas are the compounds carbamide peroxide, allantoin and Hydantoin. Ureas are closely related to biurets and structurally related to amides, carbamates, diimides, carbodiimides and thiocarbamides.
See also
Notes
- ↑ Urea. Webmineral.com. Retrieved May 15, 2007.
- ↑ Carbamide is one of the recommended International Nonproprietary Names (rINNs) used in Europe. The medicinal compound hydroxyurea (old British Approved Name) is now hydroxycarbamide.
- ↑ Birds and reptiles excrete uric acid, the product of a different form of nitrogen metabolism that requires less water.
External links
All links retrieved April 21, 2020.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
