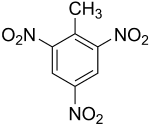
Trinitrotoluene
| Trinitrotoluene | |
|---|---|
| |
| General | |
| Name | Trinitrotoluene |
| Other Names | 2-Methyl-1,3,5-trinitrobenzene 2,4,6-Trinitrotoluene TNT Trotyl |
| Empirical formula | C7H5N3O6 |
| CAS Number | 118-96-7 |
| PubChem | 8376 |
| Short description | Pale, yellow, needle-shaped crystals |
| Characteristics | |
| Molar mass | 227.131 g/mol |
| Phase | Solid |
| Shock sensitivity | Insensitive |
| Friction sensitivity | Insensitive |
| Density | 1.654 g/cm³ |
| Explosive velocity | 6,900 m/s |
| RE factor | 1.00 |
| Melting Point | 80.35 °C |
| Boiling Point | 295 °C (Decomposition) |
| Vapor pressure | 5.7 Pa (81 °C) |
| Solubility |
|
| Safety References | |
| NFPA 704 | |
| R/S Statements |
R: 2-23/24/25-33-51/53 |
| TLV | 0.1 mg/m³ |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Trinitrotoluene (TNT) is an explosive. Its empirical formula is C7H5N3O6.
The name for TNT is, in accordance with the nomenclature of the IUPAC, 2-methyl-1,3,5-trinitrobenzene. In this article the more common designation trinitrotoluene is used.
TNT was first synthesised by Joseph Wilbrand in 1863, and the first large-scale production began in Germany in 1891.
The explosive yield of TNT is considered the standard measure of strength of bombs and other explosives (see TNT equivalent).
Characteristics
Trinitrotoluene takes the form of pale yellow, needle-shaped crystals and can be distilled in a vacuum. It is difficult to dissolve TNT in water; it is more soluble in ether, acetone, benzene and pyridine. With its low melting point of 80.35 °C, TNT can be melted in water vapour and poured into forms. TNT is poisonous and skin contact can cause allergic reactions, causing the skin to turn a bright yellow-orange color.
- Water solubility: 130 mg/L at 20 °C
- Steam pressure at 20 °C: 150 to 600 Pa
- Detonation speed: 6700-7000 m/s 6900 m/s (density: 1,6 g/cm³)
- Lead block test: 300 ml/10 g
- Sensitivity to impact: 15 N·m (1.5 kp·m)
- Friction sensitivity: to 353 N (36 kp) no reaction
Toxicity
Some military testing grounds are contaminated with TNT. Wastewater from munitions programs including contamination of surface and subsurface waters may be colored pink as the result of TNT and RDX contamination. Such contamination, called pinkwater, may be difficult and expensive to remedy.
TNT is quite toxic. It can also be absorbed through the skin, and will cause irritation and bright yellow staining. During the First World War, munition workers who handled the chemical found that their skin turned bright yellow, which led to the nickname "canary girls" or simply "canaries" to describe such workers. TNT would also eventually make ginger hair turn green. A 1916 British Government inquiry on female workers at the Royal Arsenal, Woolwich found that 37% had severe pains due to loss of appetite, nausea and constipation, 25% suffered from dermatitis, and 34% experienced changes in menstruation. Before respirators and protective grease applied to the skin were introduced, about 100 workers died from the disease.
People exposed to trinitrotoluene over a prolonged period tend to experience anemia and abnormal liver functions. Blood and liver effects, spleen enlargement and other harmful effects on the immune system have also been found in animals that ingested or breathed trinitrotoluene. There is evidence that TNT adversely affects male fertility, and TNT is listed as a possible human carcinogen. Consumption of TNT produces black urine.
History
TNT was first made in 1863 by a German chemist Joseph Wilbrand, but its potential was not seen for several years, mainly because it was so hard to detonate and because it was less powerful than other explosives. Among its advantages, however, is its ability to be safely melted using steam or hot water, allowing it to be poured molten into shell cases. (This is how Vietnamese fighters made their mines out of American shells during the Vietnam War.) It is also so insensitive that, for example, in 1910 it was exempted from the UK's Explosives Act 1875, i.e. not actually being considered an explosive for the purposes of manufacture and storage.
The German armed forces adopted it as an artillery shell filling in 1902. A particular advantage that it gave the German Navy in the First World War was being able to detonate their TNT-filled armour-piercing shells after they had penetrated the armour of British capital ships, whereas the British lyddite-filled shells tended to explode as soon as they struck the German armour, and thus expended much of their energy outside the ship. The British gradually started using it as replacement for lyddite in 1907.
Because of the insatiable demand for explosives during the Second World War, TNT was frequently mixed with 40%-80% ammonium nitrate, producing an explosive called amatol. Although nearly as powerful as TNT (and much less expensive) amatol suffered from the slight disadvantage of being hygroscopic (prone to absorbing water). Another variation called minol, consisting of amatol mixed with about 20% aluminum powder, was used by the British in mines and depth charges. Although blocks of pure TNT are available in various sizes (e.g. 250 g, 500 g and 1 kg) it is more commonly encountered in explosive blends which comprise a variable percentage of TNT plus other ingredients, e.g. torpex, tritonal, pentolite and Composition B.
Preparation
The synthesis is done in a stepwise procedure. First, toluene is nitrated with a mixture of sulfuric and nitric acid. Even lower-concentration acid mixtures are capable of doing the first and second introduction of a nitrogroup. The nitrogroups decrease the reactivity of the toluene drastically because they are electron-withdrawing groups. After separation, the mono- and dinitrotoluene is fully nitrated with a mixture of nitric acid and oleum (sulfuric acid with up to 60% dissolved SO3). This mixture is far more reactive and is capable of introducing the last nitrogroup. The waste acid from this process is used for the first step of the reaction in industrial synthesis.
See also
- dynamite
- hexanitrobenzene
- megaton
- tetryl
External links
- Computational Chemistry Wiki
- [1] Detailed Preparation
Template:ChemicalSources
bg:Тротил cs:Trinitrotoluen da:Trotyl de:Trinitrotoluol es:Trinitrotolueno eo:TNT eu:TNT fa:تیانتی fr:Trinitrotoluène ko:트라이나이트로톨루엔 id:Trinitrotoluena it:Trinitrotoluene he:TNT lv:TNT lt:TNT hu:Trinitrotoluol nl:2,4,6-trinitrotolueen ja:トリニトロトルエン no:Trinitrotoluen pl:Trotyl pt:TNT ro:Trinitrotoluen ru:Тринитротолуол simple:TNT sl:Trinitrotoluen fi:TNT sv:Trotyl tr:Trinitrotoluen zh:三硝基甲苯
