Difference between revisions of "Sewage treatment" - New World Encyclopedia
Rosie Tanabe (talk | contribs) |
|||
| Line 217: | Line 217: | ||
== External links == | == External links == | ||
| − | All links retrieved | + | All links retrieved January 26, 2023. |
*[http://www.thewatertreatments.com/category/waste-water-treatment Waste Water Treatment] - From TheWaterTreatments.com. | *[http://www.thewatertreatments.com/category/waste-water-treatment Waste Water Treatment] - From TheWaterTreatments.com. | ||
*[http://www.lagoonsonline.com Aerated Lagoons for Wastewater Treatment] - Maine Lagoons Task Force. | *[http://www.lagoonsonline.com Aerated Lagoons for Wastewater Treatment] - Maine Lagoons Task Force. | ||
| − | |||
*[https://www.epa.gov/sites/default/files/2015-06/documents/mou-green-paper-081712-v2_1.pdf Decentralized Wastewater Treatment Can Be Green and Sustainable] ''EPA''. | *[https://www.epa.gov/sites/default/files/2015-06/documents/mou-green-paper-081712-v2_1.pdf Decentralized Wastewater Treatment Can Be Green and Sustainable] ''EPA''. | ||
*[https://www.worldbank.org/en/topic/water/publication/wastewater-initiative Wastewater? From Waste to Resource] ''The World Bank''. | *[https://www.worldbank.org/en/topic/water/publication/wastewater-initiative Wastewater? From Waste to Resource] ''The World Bank''. | ||
Latest revision as of 10:11, 26 January 2023
Sewage treatment, or domestic wastewater treatment, is the process of removing contaminants from wastewater and household sewage, both runoff (effluents) and domestic. It includes processes to remove physical, chemical and biological contaminants. Its objective is to produce a waste stream (or treated effluent) and a solid waste or sludge suitable for discharge or reuse back into the environment. This material is often inadvertently contaminated with many toxic organic and inorganic compounds.
Sewage is created by residences, institutions, hospitals, and commercial and industrial establishments. It can be treated close to where it is created (in septic tanks, biofilters or aerobic treatment systems), or collected and transported via a network of pipes and pump stations to a municipal treatment plant. Sewage collection and treatment is typically subject to local, state and federal regulations and standards. Industrial sources of wastewater often require specialized treatment processes.
The sewage treatment involves three stages, called primary, secondary, and tertiary treatment. First, the solids are separated from the wastewater stream. Then, dissolved biological matter is progressively converted into a solid mass by using indigenous, water-borne micro-organisms. Finally, the biological solids are neutralized, then disposed of or re-used, and the treated water may be disinfected chemically or physically (for example by lagoons and micro-filtration). The final effluent can be discharged into a stream, river, bay, lagoon or wetland, or it can be used for the irrigation of a golf course, green way or park. If it is sufficiently clean, it can also be used for groundwater recharge or agricultural purposes.
Description
Raw influent (sewage) includes household waste liquid from toilets, baths, showers, kitchens, sinks, and so forth that is disposed of via sewers. In many areas, sewage also includes liquid waste from industry and commerce.
The separation and draining of household waste into greywater and blackwater is becoming more common in the developed world, with greywater being permitted to be used for watering plants or recycled for flushing toilets. A lot of sewage also includes some surface water from roofs or hard-standing areas. Municipal wastewater therefore includes residential, commercial, and industrial liquid waste discharges, and may include stormwater runoff. Sewage systems capable of handling stormwater are known as combined systems or combined sewers. Such systems are usually avoided since they complicate and thereby reduce the efficiency of sewage treatment plants owing to their seasonality. The variability in flow also leads to often larger than necessary, and subsequently more expensive, treatment facilities. In addition, heavy storms that contribute more flows than the treatment plant can handle may overwhelm the sewage treatment system, causing a spill or overflow (called a combined sewer overflow, or CSO, in the United States). It is preferable to have a separate storm drain system for stormwater in areas that are developed with sewer systems.
As rainfall runs over the surface of roofs and the ground, it may pick up various contaminants including soil particles and other sediment, heavy metals, organic compounds, animal waste, and oil and grease. Some jurisdictions require stormwater to receive some level of treatment before being discharged directly into waterways. Examples of treatment processes used for stormwater include sedimentation basins, wetlands, buried concrete vaults with various kinds of filters, and vortex separators (to remove coarse solids).
The site where the raw wastewater is processed before it is discharged back to the environment is called a wastewater treatment plant (WWTP). The order and types of mechanical, chemical and biological systems that comprise the wastewater treatment plant are typically the same for most developed countries:
- Mechanical treatment
- Influx (Influent)
- Removal of large objects
- Removal of sand and grit
- Pre-precipitation
- Biological treatment
- Oxidation bed (oxidizing bed) or aeration system
- Post precipitation
- Chemical treatment this step is usually combined with settling and other processes to remove solids, such as filtration. The combination is referred to in the United States as physical chemical treatment.
Primary treatment removes the materials that can be easily collected from the raw wastewater and disposed of. The typical materials that are removed during primary treatment include fats, oils, and greases (also referred to as FOG), sand, gravels and rocks (also referred to as grit), larger settleable solids and floating materials (such as rags and flushed feminine hygiene products). This step is done entirely with machinery.
Primary treatment
Removal of large objects from influent sewage
In primary treatment, the influent sewage water is strained to remove all large objects that are deposited in the sewer system, such as rags, sticks, tampons, cans, fruit, etc. This is most commonly done with a manual or automated mechanically raked bar screen. The raking action of a mechanical bar screen is typically paced according to the accumulation on the bar screens and/or flow rate. The bar screen is used because large solids can damage or clog the equipment used later in the sewage treatment plant. The solids are collected in a dumpster and later disposed in a landfill.
Primary treatment also typically includes a sand or grit channel or chamber where the velocity of the incoming wastewater is carefully controlled to allow sand grit and stones to settle, while keeping the majority of the suspended organic material in the water column. This equipment is called a degritter or sand catcher. Sand, grit, and stones need to be removed early in the process to avoid damage to pumps and other equipment in the remaining treatment stages. Sometimes there is a sand washer (grit classifier) followed by a conveyor that transports the sand to a container for disposal. The contents from the sand catcher may be fed into the incinerator in a sludge processing plant, but in many cases, the sand and grit is sent to a landfill.
Sedimentation
Many plants have a sedimentation stage where the sewage is allowed to pass slowly through large tanks, commonly called "primary clarifiers" or "primary sedimentation tanks." The tanks are large enough that sludge can settle and floating material such as grease and oils can rise to the surface and be skimmed off. The main purpose of the primary clarification stage is to produce both a generally homogeneous liquid capable of being treated biologically and a sludge that can be separately treated or processed. Primary settling tanks are usually equipped with mechanically driven scrapers that continually drive the collected sludge towards a hopper in the base of the tank from where it can be pumped to further sludge treatment stages.
Secondary treatment
Secondary treatment is designed to substantially degrade the biological content of the sewage such as are derived from human waste, food waste, soaps and detergent. The majority of municipal plants treat the settled sewage liquor using aerobic biological processes. For this to be effective, the biota require both oxygen and a substrate on which to live. There are a number of ways in which this is done. In all these methods, the bacteria and protozoa consume biodegradable soluble organic contaminants (e.g. sugars, fats, organic short-chain carbon molecules, etc.) and bind much of the less soluble fractions into floc. Secondary treatment systems are classified as fixed film or suspended growth. Fixed-film treatment process including trickling filter and rotating biological contactors where the biomass grows on media and the sewage passes over its surface. In suspended growth systems—such as activated sludge—the biomass is well mixed with the sewage and can be operated in a smaller space than fixed-film systems that treat the same amount of water. However, fixed-film systems are more able to cope with drastic changes in the amount of biological material and can provide higher removal rates for organic material and suspended solids than suspended growth systems.
Roughing filters are intended to treat particularly strong or variable organic loads, typically industrial, to allow them to then be treated by conventional secondary treatment processes. Characteristics include typically tall, circular filters filled with open synthetic filter media to which wastewater is applied at a relatively high rate. They are designed to allow high hydraulic loading and a high flow-through of air. On larger installations, air is forced through the media using blowers. The resultant wastewater is usually within the normal range for conventional treatment processes.
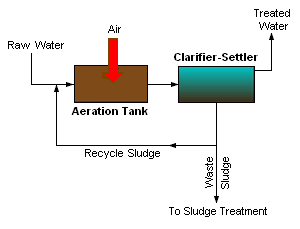
Activated sludge
In general, activated sludge plants encompass a variety of mechanisms and processes that use dissolved oxygen to promote the growth of biological floc that substantially removes organic material.
The process traps particulate material and can, under ideal conditions, convert ammonia to nitrite and nitrate and ultimately to nitrogen gas, (see also denitrification).
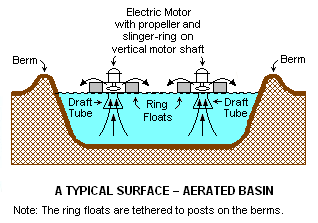
Surface-aerated basins
Most biological oxidation processes for treating industrial wastewaters have in common the use of oxygen (or air) and microbial action. Surface-aerated basins achieve 80 to 90 percent removal of Biochemical Oxygen Demand with retention times of 1 to 10 days.[1] The basins may range in depth from 1.5 to 5.0 meters and use motor-driven aerators floating on the surface of the wastewater.[1]
In an aerated basin system, the aerators provide two functions: they transfer air into the basins required by the biological oxidation reactions, and they provide the mixing required for dispersing the air and for contacting the reactants (that is, oxygen, wastewater and microbes). Typically, the floating surface aerators are rated to deliver the amount of air equivalent to 1.8 to 2.7 kg O2/kW•h. However, they do not provide as good mixing as is normally achieved in activated sludge systems and therefore aerated basins do not achieve the same performance level as activated sludge units.[1]
Biological oxidation processes are sensitive to temperature and, between 0 °C and 40 °C, the rate of biological reactions increase with temperature. Most surface aerated vessels operate at between 4 °C and 32 °C.[1]
Fluidized bed reactors
The carbon absorption following biological treatment is particularly effective in reducing both the BOD and COD to low levels. A fluidized bed reactor is a combination of the most common stirred tank packed bed, continuous flow reactors. It is very important to chemical engineering because of its excellent heat and mass transfer characteristics. In a fluidized bed reactor, the substrate is passed upward through the immobilized enzyme bed at a high velocity to lift the particles. However the velocity must not be so high that the enzymes are swept away from the reactor entirely. This causes low mixing; these type of reactors are highly suitable for the exothermic reactions. It is most often applied in immobilized enzyme catalysis
Filter beds (oxidizing beds)
In older plants and plants receiving more variable loads, trickling filter beds are used where the settled sewage liquor is spread onto the surface of a deep bed made up of coke (carbonized coal), limestone chips or specially fabricated plastic media. Such media must have high surface areas to support the biofilms that form. The liquor is distributed through perforated rotating arms radiating from a central pivot. The distributed liquor trickles through this bed and is collected in drains at the base. These drains also provide a source of air which percolates up through the bed, keeping it aerobic. Biological films of bacteria, protozoa and fungi form on the media’s surfaces and eat or otherwise reduce the organic content. This biofilm is grazed by insect larvae and worms which help maintain an optimal thickness. Overloading of beds increases the thickness of the film leading to clogging of the filter media and ponding on the surface.
Biological aerated filters
Biological Aerated (or Anoxic) Filter (BAF) or Biofilters combine filtration with biological carbon reduction, nitrification or denitrification. BAF usually includes a reactor filled with a filter media. The media is either in suspension or supported by a gravel layer at the foot of the filter. The dual purpose of this media is to support highly active biomass that is attached to it and to filter suspended solids. Carbon reduction and ammonia conversion occurs in aerobic mode and sometime achieved in a single reactor while nitrate conversion occurs in anoxic mode. BAF is operated either in upflow or downflow configuration depending on design specified by manufacturer.
Membrane bioreactors
Membrane bioreactors (MBR) combines activated sludge treatment with a membrane liquid-solid separation process. The membrane component uses low pressure microfiltration or ultra filtration membranes and eliminates the need for clarification and tertiary filtration. The membranes are typically immersed in the aeration tank (however, some applications utilize a separate membrane tank). One of the key benefits of a membrane bioreactor system is that it effectively overcomes the limitations associated with poor settling of sludge in conventional activated sludge (CAS) processes. The technology permits bioreactor operation with considerably higher mixed liquor suspended solids (MLSS) concentration than CAS systems, which are limited by sludge settling. The process is typically operated at MLSS in the range of 8,000–12,000 mg/L, while CAS are operated in the range of 2,000–3,000 mg/L. The elevated biomass concentration in the membrane bioreactor process allows for very effective removal of both soluble and particulate biodegradable materials at higher loading rates. Thus increased Sludge Retention Times (SRTs)—usually exceeding 15 days—ensure complete nitrification even in extremely cold weather.
The cost of building and operating a MBR is usually higher than conventional wastewater treatment, however, as the technology has become increasingly popular and has gained wider acceptance throughout the industry, the life-cycle costs have been steadily decreasing. The small footprint of MBR systems, and the high quality effluent produced, makes them particularly useful for water reuse applications.
Secondary sedimentation
The final step in the secondary treatment stage is to settle out the biological floc or filter material and produce sewage water containing very low levels of organic material and suspended matter.
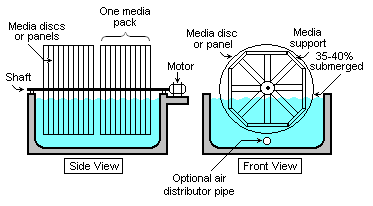
Rotating biological contactors
Rotating biological contactors (RBCs) are mechanical secondary treatment systems, which are robust and capable of withstanding surges in organic load. RBCs were first installed in Germany in 1960 and have since been developed and refined into a reliable operating unit. The rotating disks support the growth of bacteria and micro-organisms present in the sewage, which breakdown and stabilize organic pollutants. To be successful, micro-organisms need both oxygen to live and food to grow. Oxygen is obtained from the atmosphere as the disks rotate. As the micro-organisms grow, they build up on the media until they are sloughed off due to shear forces provided by the rotating discs in the sewage. Effluent from the RBC is then passed through final clarifiers where the micro-organisms in suspension settle as a sludge. The sludge is withdrawn from the clarifier for further treatment.
A functionally similar biological filtering system has become popular as part of home aquarium filtration and purification. The aquarium water is drawn up out of the tank and then cascaded over a freely spinning corrugated fiber-mesh wheel before passing through a media filter and back into the aquarium. The spinning mesh wheel develops a biofilm coating of microorganisms that feed on the suspended wastes in the aquarium water and are also exposed to the atmosphere as the wheel rotates. This is especially good at removing waste urea and ammonia urinated into the aquarium water by the fish and other animals.
Tertiary treatment
The purpose of tertiary treatment is to provide a final treatment stage to raise the effluent quality before it is discharged to the receiving environment (sea, river, lake, ground, etc.). More than one tertiary treatment process may be used at any treatment plant. If disinfection is practiced, it is always the final process. It is also called "effluent polishing."
Filtration
Sand filtration removes much of the residual suspended matter. Filtration over activated carbon removes residual toxins.
Lagooning
Lagooning provides settlement and further biological improvement through storage in large man-made ponds or lagoons. These lagoons are highly aerobic and colonization by native macrophytes, especially reeds, is often encouraged. Small filter feeding invertebrates such as Daphnia and species of Rotifera greatly assist in treatment by removing fine particulates.
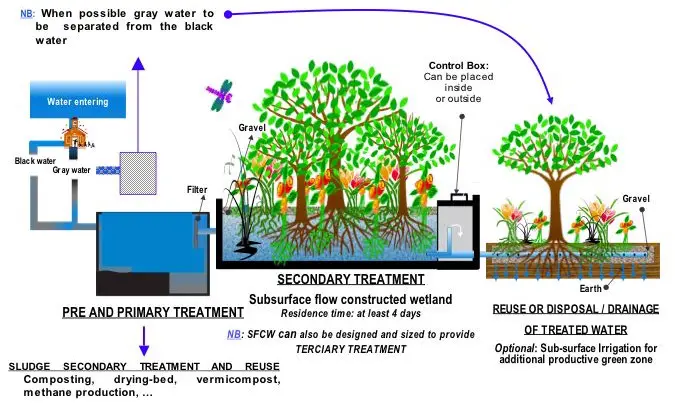
Constructed wetlands
Constructed wetlands include engineered reedbeds and a range of similar methodologies, all of which provide a high degree of aerobic biological improvement and can often be used instead of secondary treatment for small communities, also see phytoremediation. One example is a small reedbed used to clean the drainage from the elephants' enclosure at Chester Zoo in England.
Nutrient removal
Wastewater may contain high levels of the nutrients nitrogen and phosphorus. Excessive release to the environment can lead to a build up of nutrients, called eutrophication, which can in turn encourage the overgrowth of weeds, algae, and cyanobacteria (blue-green algae). This may cause an algal bloom, a rapid growth in the population of algae. The algae numbers are unsustainable and eventually most of them die. The decomposition of the algae by bacteria uses up so much of oxygen in the water that most or all of the animals die, which creates more organic matter for the bacteria to decompose. In addition to causing deoxygenation, some algal species produce toxins that contaminate drinking water supplies. Different treatment processes are required to remove nitrogen and phosphorus.
Nitrogen removal
The removal of nitrogen is effected through the biological oxidation of nitrogen from ammonia (nitrification) to nitrate, followed by denitrification, the reduction of nitrate to nitrogen gas. Nitrogen gas is released to the atmosphere and thus removed from the water.
Nitrification itself is a two-step aerobic process, each step facilitated by a different type of bacteria. The oxidation of ammonia (NH3) to nitrite (NO2−) is most often facilitated by Nitrosomonas spp. (nitroso referring to the formation of a nitroso functional group). Nitrite oxidation to nitrate (NO3−), though traditionally believed to be facilitated by Nitrobacter spp. (nitro referring the formation of a nitro functional group), is now known to be facilitated in the environment almost exclusively by Nitrospira spp.
Denitrification requires anoxic conditions to encourage the appropriate biological communities to form. It is facilitated by a wide diversity of bacteria. Sand filters, lagooning and reed beds can all be used to reduce nitrogen, but the activated sludge process (if designed well) can do the job the most easily. Since denitrification is the reduction of nitrate to dinitrogen gas, an electron donor is needed. This can be, depending on the wastewater, organic matter (from faeces), sulfide, or an added donor like methanol.
Sometimes the conversion of toxic ammonia to nitrate alone is referred to as tertiary treatment.
Phosphorus removal
Phosphorus removal is important as it is a limiting nutrient for algae growth in many fresh water systems (for negative effects of algae see Nutrient removal). It is also particularly important for water reuse systems where high phosphorus concentrations may lead to fouling of downstream equipment such as reverse osmosis.
Phosphorus can be removed biologically in a process called enhanced biological phosphorus removal. In this process, specific bacteria, called polyphosphate accumulating organisms (PAOs), are selectively enriched and accumulate large quantities of phosphorus within their cells (up to 20 percent of their mass). When the biomass enriched in these bacteria is separated from the treated water, these biosolids have a high fertilizer value.
Phosphorus removal can also be achieved by chemical precipitation, usually with salts of iron (e.g. ferric chloride), aluminum (e.g. alum), or lime. This may lead to excessive sludge productions as hydroxides precipitates and the added chemicals can be expensive. Despite this, chemical phosphorus removal requires significantly smaller equipment footprint than biological removal, is easier to operate and is often more reliable than biological phosphorus removal.
Once removed, phosphorus, in the form of a phosphate rich sludge, may be land filled or, if in suitable condition, resold for use in fertilizer.
Disinfection
The purpose of disinfection in the treatment of wastewater is to substantially reduce the number of microorganisms in the water to be discharged back into the environment. The effectiveness of disinfection depends on the quality of the water being treated (e.g., cloudiness, pH, etc.), the type of disinfection being used, the disinfectant dosage (concentration and time), and other environmental variables. Cloudy water will be treated less successfully since solid matter can shield organisms, especially from ultraviolet light or if contact times are low. Generally, short contact times, low doses and high flows all militate against effective disinfection. Common methods of disinfection include ozone, chlorine, or ultraviolet light. Chloramine, which is used for drinking water, is not used in wastewater treatment because of its persistence.
Chlorination remains the most common form of wastewater disinfection in North America due to its low cost and long-term history of effectiveness. One disadvantage is that chlorination of residual organic material can generate chlorinated-organic compounds that may be carcinogenic or harmful to the environment. Residual chlorine or chloramines may also be capable of chlorinating organic material in the natural aquatic environment. Further, because residual chlorine is toxic to aquatic species, the treated effluent must also be chemically dechlorinated, adding to the complexity and cost of treatment.
Ultraviolet (UV) light can be used instead of chlorine, iodine, or other chemicals. Because no chemicals are used, the treated water has no adverse effect on organisms that later consume it, as may be the case with other methods. UV radiation causes damage to the genetic structure of bacteria, viruses, and other pathogens, making them incapable of reproduction. The key disadvantages of UV disinfection are the need for frequent lamp maintenance and replacement and the need for a highly treated effluent to ensure that the target microorganisms are not shielded from the UV radiation (i.e., any solids present in the treated effluent may protect microorganisms from the UV light). In the United Kingdom, light is becoming the most common means of disinfection because of the concerns about the impacts of chlorine in chlorinating residual organics in the wastewater and in chlorinating organics in the receiving water. Edmonton, Alberta, Canada also uses UV light for its water treatment.
Ozone O3 is generated by passing oxygen O2 through a high voltage potential resulting in a third oxygen atom becoming attached and forming O3. Ozone is very unstable and reactive and oxidizes most organic material it comes in contact with, thereby destroying many pathogenic microorganisms. Ozone is considered to be safer than chlorine because, unlike chlorine which has to be stored on site (highly poisonous in the event of an accidental release), ozone is generated onsite as needed. Ozonation also produces fewer disinfection by-products than chlorination. A disadvantage of ozone disinfection is the high cost of the ozone generation equipment and the requirements for special operators.
Package plants and batch reactors
In order to use less space, treat difficult waste, deal with intermittent flow or achieve higher environmental standards, a number of designs of hybrid treatment plants have been produced. Such plants often combine all or at least two stages of the three main treatment stages into one combined stage. In the UK, where a large number of sewage treatment plants serve small populations, package plants are a viable alternative to building discrete structures for each process stage.
One type of system that combines secondary treatment and settlement is the sequencing batch reactor (SBR). Typically, activated sludge is mixed with raw incoming sewage and mixed and aerated. The resultant mixture is then allowed to settle producing a high quality effluent. The settled sludge is run off and re-aerated before a proportion is returned to the head of the works. SBR plants are now being deployed in many parts of the world including North Liberty, Iowa, and Llanasa, North Wales.
The disadvantage of such processes is that precise control of timing, mixing and aeration is required. This precision is usually achieved by computer controls linked to many sensors in the plant. Such a complex, fragile system is unsuited to places where such controls may be unreliable, or poorly maintained, or where the power supply may be intermittent.
Package plants may be referred to as high charged or low charged. This refers to the way the biological load is processed. In high charged systems, the biological stage is presented with a high organic load and the combined floc and organic material is then oxygenated for a few hours before being charged again with a new load. In the low charged system the biological stage contains a low organic load and is combined with flocculate for a relatively long time.
Sludge treatment and disposal
The sludges accumulated in a wastewater treatment process must be treated and disposed of in a safe and effective manner. The purpose of digestion is to reduce the amount of organic matter and the number of disease-causing microorganisms present in the solids. The most common treatment options include anaerobic digestion, aerobic digestion, and composting.
choice of a wastewater solid treatment method depends on the amount of solids generated and other site-specific conditions. However, in general, composting is most often applied to smaller-scale applications followed by aerobic digestion and then lastly anaerobic digestion for the larger-scale municipal applications.
Anaerobic digestion
Anaerobic digestion is a bacterial process that is carried out in the absence of oxygen. The process can either be thermophilic digestion, in which sludge is fermented in tanks at a temperature of 55°C, or mesophilic, at a temperature of around 36°C. Though allowing shorter retention time (and thus smaller tanks), thermophilic digestion is more expensive in terms of energy consumption for heating the sludge.
One major feature of anaerobic digestion is the production of biogas, which can be used in generators for electricity production and/or in boilers for heating purposes.
Aerobic digestion
Aerobic digestion is a bacterial process occurring in the presence of oxygen. Under aerobic conditions, bacteria rapidly consume organic matter and convert it into carbon dioxide. The operating costs used to be characteristically much greater for aerobic digestion because of the energy used by the blowers, pumps, and motors needed to add oxygen to the process. However, recent technological advances include non-electric aerated filter systems that use natural air currents for the aeration instead of electrically operated machinery. Aerobic digestion can also be achieved by using diffuser systems or jet aerators to oxidize the sludge.
Composting
Composting is also an aerobic process that involves mixing the sludge with sources of carbon such as sawdust, straw or wood chips. In the presence of oxygen, bacteria digest both the wastewater solids and the added carbon source and, in doing so, produce a large amount of heat.
Sludge disposal
When a liquid sludge is produced, further treatment may be required to make it suitable for final disposal. Typically, sludges are thickened (dewatered) to reduce the volumes transported off-site for disposal. There is no process which completely eliminates the need to dispose of biosolids. There is, however, an additional step some cities are taking to superheat the wastewater sludge and convert it into small pelletized granules that are high in nitrogen and other organic materials. In New York City, for example, several sewage treatment plants have dewatering facilities that use large centrifuges along with the addition of chemicals such as polymer to further remove liquid from the sludge. The removed fluid, called centrate, is typically reintroduced into the wastewater process. The product which is left is called "cake" and that is picked up by companies which turn it into fertilizer pellets. This product is then sold to local farmers and turf farms as a soil amendment or fertilizer, reducing the amount of space required to dispose of sludge in landfills.[2]
Treatment in the receiving environment
Many processes in a wastewater treatment plant are designed to mimic the natural treatment processes that occur in the environment, whether that environment is a natural water body or the ground. If not overloaded, bacteria in the environment will consume organic contaminants, although this will reduce the levels of oxygen in the water and may significantly change the overall ecology of the receiving water. Native bacterial populations feed on the organic contaminants, and the numbers of disease-causing microorganisms are reduced by natural environmental conditions such as predation exposure to ultraviolet radiation, for example. Consequently, in cases where the receiving environment provides a high level of dilution, a high degree of wastewater treatment may not be required. However, recent evidence has demonstrated that very low levels of certain contaminants in wastewater, including hormones (from animal husbandry and residue from human hormonal contraception methods) and synthetic materials such as phthalates that mimic hormones in their action, can have an unpredictable adverse impact on the natural biota and potentially on humans if the water is re-used for drinking water. In the United States and EU, uncontrolled discharges of wastewater to the environment are not permitted under law, and strict water quality requirements are to be met. A significant threat in the coming decades will be the increasing uncontrolled discharges of wastewater within rapidly developing countries.
Sewage treatment in developing countries
There are few reliable figures on the share of the wastewater collected in sewers that is being treated in the world. In many developing countries the bulk of domestic and industrial wastewater is discharged without any treatment or after primary treatment only. Sewage treatment rates are highly unequal for different countries around the world. For example, while high-income countries treat approximately 74 percent of their sewage, developing countries treat an average of just 4 percent.[3]
In a relatively developed Middle Eastern country such as Iran, Tehran's majority of population has totally untreated sewage injected to the city’s groundwater.[4] Israel has also aggressively pursued the use of treated sewer water for irrigation. The country treats and reuses 90 percent of its wastewater, transporting treated wastewater to farms all across the country:
Israel’s wastewater, which is funneled primarily through Shafdan, is treated in a multi-step process that includes basic filtration and biological treatments and ends with a natural form of filtration called soil aquifer treatment (SAT). SAT allows the country’s abundant sand to filter remaining pollutants from the water – a six-month to one-year filtration process which deposits the water in an agriculture-specific aquifer beneath the filtration fields.[5]
Most of sub-Saharan Africa is without wastewater treatment.
Water utilities in developing countries are chronically underfunded because of low water tariffs, the nonexistence of sanitation tariffs in many cases, low billing efficiency (i.e. many users that are billed do not pay) and poor operational efficiency (i.e. there are overly high levels of staff, there are high physical losses, and many users have illegal connections and are thus not being billed). In addition, wastewater treatment typically is the process within the utility that receives the least attention, partly because enforcement of environmental standards is poor. As a result of all these factors, operation and maintenance of many wastewater treatment plants is poor. This is evidenced by the frequent breakdown of equipment, shutdown of electrically operated equipment due to power outages or to reduce costs, and sedimentation due to lack of sludge removal.
Developing countries as diverse as Egypt, Algeria, China or Colombia have invested substantial sums in wastewater treatment without achieving a significant impact in terms of environmental improvement. Even if wastewater treatment plants are properly operating, it can be argued that the environmental impact is limited in cases where the assimilative capacity of the receiving waters (ocean with strong currents or large rivers) is high, as it is often the case.
Benefits of wastewater treatment compared to benefits of sewage collection in developing countries
Waterborne diseases that are prevalent in developing countries, such as typhus and cholera, are caused primarily by poor hygiene practices and the absence of improved household sanitation facilities. The public health impact of the discharge of untreated wastewater is comparatively much lower. Hygiene promotion, on-site sanitation and low-cost sanitation thus are likely to have a much greater impact on public health than wastewater treatment.
See also
Notes
- ↑ 1.0 1.1 1.2 1.3 M.R. Beychok, Performance of surface-aerated basins Chemical Engineering Progress Symposium Series 67(107) (1971):322–339. Retrieved March 8, 2022.
- ↑ Understand Biosolids: Fertilizer and Fuel New England Fertilizer Company (NEFCO). Retrieved March 8, 2022.
- ↑ Edward R. Jones, Michelle T. H. van Vliet, Manzoor Qadir, and Marc F. P. Bierkens, Country-level and gridded estimates of wastewater production, collection, treatment and reuse Earth System Science Data 13(2) (February 8, 2021):237–254. Retrieved March 8, 2022.
- ↑ Massoud Tajrishy and Ahmad Abrishamchi, "Integrated Approach to Water and Wastewater Management for Tehran, Iran," in Water Conservation, Reuse, and Recycling: Proceedings of the Iranian-American Workshop (Washington, DC: National Academy Press, 2014, ISBN 978-0309545020).
- ↑ Karyn Simpson, What the world can learn from Israel’s water reuse programs Medill Reports, October 18, 2018. Retrieved March 8, 2022.
ReferencesISBN links support NWE through referral fees
- Committee on U.S-Iranian Workshop on Water Conservation, Reuse, and Recycling. Water Conservation, Reuse, and Recycling: Proceedings of the Iranian-American Workshop. Washington, DC: National Academies Press, 2005. ISBN 9780309545020
- Drinan, Joanne E. Water and Wastewater Treatment: A Guide for the Nonengineering Professionals. Boca Raton, FL: Lewis Publishers, 2000. ISBN 1587160498.
- Droste, Ronald L. Theory and Practice of Water and Wastewater Treatment. New York, NY: J. Wiley, 1997. ISBN 0471124443.
- National Research Council, Water Conservation, Reuse, and Recycling: Proceedings of an Iranian-American Workshop. National Academy Press, 2014. ISBN 978-0309545020
- Spellman, Frank R. Handbook of Water and Wastewater Treatment Plant Operations, Second ed. Boca Raton, FL: CRC Press/Taylor & Francis, 2009. ISBN 1420075306.
External links
All links retrieved January 26, 2023.
- Waste Water Treatment - From TheWaterTreatments.com.
- Aerated Lagoons for Wastewater Treatment - Maine Lagoons Task Force.
- Decentralized Wastewater Treatment Can Be Green and Sustainable EPA.
- Wastewater? From Waste to Resource The World Bank.
- Sustainable Wastewater Management Methods by Jane Marsh, Environment, June 30, 2021.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.