Difference between revisions of "Pyruvic acid" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 19: | Line 19: | ||
}} | }} | ||
}} | }} | ||
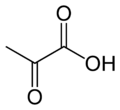
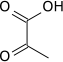
| − | '''Pyruvic acid''' | + | '''Pyruvic acid''' is a three-carbon, [[keto acids|alpha-keto acid]] that plays an important role in biochemical processes. It has the chemical formula CH<sub>3</sub>COCO<sub>2</sub>H. The [[carboxylate]] [[anion]] of pyruvic acid is known as '''pyruvate'''. |
| + | |||
| + | Pyruvic acid is formed as an end product of [[glycolysis]], which breaks down [[glucose]] (a six-carbon-molecule) into [[pyruvate]] (a three-carbon molecule). This not only produces a small net gain of the universal energy storage molecule [[Adenosine_triphosphate|adenosine triphosphate]] (ATP), to power cellular function, but as part of [[aerobic respiration]] also provides pyruvate that can be converted to acetyl-CoA ([[acetyl coenzyme A]]) for the critically important [[citric acid cycle]] (also known as the Krebs cycle). In the absence of oxygen, pyruvate can be reduce to [[lactic acid]]. | ||
| + | |||
| + | intricacy | ||
==Chemistry== | ==Chemistry== | ||
Revision as of 20:14, 17 May 2008
| Pyruvic acid | |
|---|---|
|
|
| IUPAC name | 2-oxopropanoic acid |
| Other names | α-ketopropionic acid; acetylformic acid; pyroracemic acid; Pyr |
| Identifiers | |
| CAS number | [] |
| SMILES | CC(C(O)=O)=O |
| Properties | |
| Molecular formula | C3H4O3 |
| Molar mass | 88.06 g/mol |
| Density | 1.250 g/cm³ |
| Melting point |
11.8 °C |
| Boiling point |
165 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Pyruvic acid is a three-carbon, alpha-keto acid that plays an important role in biochemical processes. It has the chemical formula CH3COCO2H. The carboxylate anion of pyruvic acid is known as pyruvate.
Pyruvic acid is formed as an end product of glycolysis, which breaks down glucose (a six-carbon-molecule) into pyruvate (a three-carbon molecule). This not only produces a small net gain of the universal energy storage molecule adenosine triphosphate (ATP), to power cellular function, but as part of aerobic respiration also provides pyruvate that can be converted to acetyl-CoA (acetyl coenzyme A) for the critically important citric acid cycle (also known as the Krebs cycle). In the absence of oxygen, pyruvate can be reduce to lactic acid.
intricacy
Chemistry
Carboxylic acids are organic acids characterized by the presence of one or more carboxyl groups in their molecules. A carboxyl group consists of a carbon atom attached to an oxygen atom with a double covalent bond and to a hydroxyl group by a single covalent bond. The chemical formula of the carboxyl group may be written as -C(=O)OH, -COOH, or -CO2H.[1] Salts and anions of carboxylic acids are called carboxylates.
Keto acids are organic acids containing a ketone functional group and a carboxylic acid group.
Common types of keto acids include:
- Alpha-keto acids, or 2-oxo acids, such as pyruvic acid have the keto group adjacent to the carboxylic acid
Pyruvic acid is a colorless liquid with a smell similar to that of acetic acid. It is miscible with water, and soluble in ethanol and diethyl ether. In the laboratory, pyruvic acid may be prepared by heating a mixture of tartaric acid and potassium hydrogen sulfate, or by the hydrolysis of acetyl cyanide, formed by reaction of acetyl chloride with potassium cyanide:
- CH3COCl + KCN → CH3COCN
- CH3COCN → CH3COCOOH
Biochemistry
Pyruvate is an important chemical compound in biochemistry. It is the output of the aerobic metabolism of glucose known as glycolysis. One molecule of glucose breaks down into two molecules of pyruvate, which are then used to provide further energy, in one of two ways. Pyruvate is converted into acetyl-coenzyme A, which is the main input for a series of reactions known as the Krebs cycle. Pyruvate is also converted to oxaloacetate by an anaplerotic reaction which replenishes Krebs cycle intermediates; alternatively, the oxaloacetate is used for gluconeogenesis. These reactions are named after Hans Adolf Krebs, the biochemist awarded the 1953 Nobel Prize for physiology, jointly with Fritz Lipmann, for research into metabolic processes. The cycle is also called the citric acid cycle, because citric acid is one of the intermediate compounds formed during the reactions.
- In carbohydrate catabolism (the breakdown of sugars), the citric acid cycle follows glycolysis, which breaks down glucose (a six-carbon-molecule) into pyruvate (a three-carbon molecule). In eukaryotes, pyruvate moves into the mitochondria. It is converted into acetyl-CoA (acetyl coenzyme A) and enters the citric acid cycle.
If insufficient oxygen is available, the acid is broken down anaerobically, creating lactic acid in animals and ethanol in plants. Pyruvate from glycolysis is converted by anaerobic respiration to lactate using the enzyme lactate dehydrogenase and the coenzyme NADH in lactate fermentation, or to acetaldehyde and then to ethanol in alcoholic fermentation.
In glycolysis, the six-carbon sugar glucose (Glc) is oxidized to two molecules of pyruvic acid (Pyr), yielding a small net gain of chemical energy (ATP) to power cellular function.
Pyruvate is a key intersection in the network of metabolic pathways. Pyruvate can be converted to carbohydrates via gluconeogenesis, to fatty acids or energy through acetyl-CoA, to the amino acid alanine and to ethanol. Therefore it unites several key metabolic processes.
The pyruvic acid derivative bromopyruvic acid is being studied for potential cancer treatment applications by researchers at Johns Hopkins University in ways that would support the Warburg hypothesis on the cause(s) of cancer.
Pyruvate production by glycolysis
Glycolysis is a series of biochemical reactions by which one molecule of glucose (Glc) is oxidized to two molecules of pyruvic acid (Pyr) an a relatively small amount of the universal energy storage molecule adenosine triphosphate (ATP). This breakdown of the simple sugar glucose serves three principal functions:
- Generation of the high-energy molecules (ATP and NADH), which are used as cellular energy sources in both aerobic respiration (with oxygen) and anaerobic respiration (without oxygen)
- Production of pyruvate for the citric acid cycle as part of aerobic respiration
- Production of a variety of six- or three-carbon intermediate metabolites, which may be removed at various steps in the process for other cellular purposes (such as nucleotide biosynthesis).
As the foundation of both aerobic and anaerobic respiration, glycolysis is the archetype of universal metabolic processes known and occurring (with variations) in many types of cells in nearly all organisms. Glycolysis, through anaerobic respiration, is the main energy source in many prokaryotes, eukaryotic cells devoid of mitochondria (e.g., mature erythrocytes), and eukaryotic cells under low-oxygen conditions (e.g., heavily-exercising muscle or fermenting yeast). The near ubiquity of these reactions suggests harmony and connectivity among organisms and great antiquity; glycolysis may have originated with the first prokaryotes (organisms without a cell nucleus) at least 3.5 billion years ago.
In glycolysis, phosphoenolpyruvate (PEP) is converted to pyruvate by pyruvate kinase. This reaction is strongly exergonic and irreversible; in gluconeogenesis it takes two enzymes, pyruvate carboxylase and PEP carboxykinase to catalyze the reverse transformation of pyruvate to PEP. The arrow indicating a reverse reaction in the Figure below is incorrect.
| phosphoenolpyruvate | Pyruvate kinase | pyruvate | |
|
| ||
| ADP | ATP | ||
| |||
| ADP | ATP | ||
| Pyruvate kinase | |||
Compound C00074 at KEGG Pathway Database. Enzyme 2.7.1.40 at KEGG Pathway Database. Compound C00022 at KEGG Pathway Database.
Pyruvate decarboxylation to acetyl CoA
Pyruvate decarboxylation by the pyruvate dehydrogenase complex produces acetyl-CoA.
| pyruvate | pyruvate dehydrogenase complex | acetyl-CoA | |

|
|||
| CoA + NAD+ | CO2 + NADH + H+ | ||
| |||
Pyruvate carboxylation to oxaloacetate
Carboxylation by the pyruvate carboxylase produces oxaloacetate.
| pyruvate | pyruvate carboxylase | oxaloacetate | |

|
| ||
| ATP + CO2 | ADP + Pi | ||

| |||
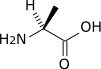
Transamination by the alanine aminotransferase
| pyruvate | Alanine transaminase | alanine | |

|
| ||
| Glutamate | α-ketoglutarate | ||
| |||
| Glutamate | α-ketoglutarate | ||
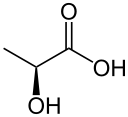
Reduction to lactate
Reduction by the lactate dehydrogenase produces lactate.
| pyruvate | lactate dehydrogenase | lactate | |

|
| ||
| NADH | NAD+ | ||
| |||
| NADH | NAD+ | ||
Origin of life
Current evolutionary theory on the origin of life posits that the first organisms were anaerobic because the atmosphere of prebiotic Earth was almost devoid of oxygen. As such, requisite biochemical materials must have preceded life and recent experiments indicate that pyruvate can be synthesized abiotically. In vitro, iron sulfide at sufficient pressure and temperature catalyzes the formation of pyruvate. Thus, argues Günter Wächtershäuser, the mixing of iron-rich crust with hydrothermal vent fluid is suspected of providing the fertile basis for the formation of life.
ReferencesISBN links support NWE through referral fees
- George D. Cody, Nabil Z. Boctor, Timothy R. Filley, Robert M. Hazen, James H. Scott, Anurag Sharma, Hatten S. Yoder Jr., "Primordial Carbonylated Iron-Sulfur Compounds and the Synthesis of Pyruvate," Science, 289 (5483) (25 August 2000) pp. 1337 - 1340. [1]
External links
- "Pyruvate in Cancer Prevention and Treatment"
- "ENERGY BLOCKER" KILLS BIG TUMORS IN RATS, research at Johns Hopkins on pyruvate
- "Latin uva grapes"
| Glycolysis Metabolic Pathway | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ Compendium of Chemical Terminology, carboxylic acids Retrieved December 8, 2007.