Nitrogen cycle
The nitrogen cycle is the biogeochemical cycle that describes the transformations of nitrogen and nitrogen-containing compounds in nature.
The basic Earth's atmosphere is about 78% nitrogen, making it the largest pool of nitrogen. Nitrogen is essential for many biological processes; it is in all amino acids, is incorporated into proteins, and is present in the bases that make up nucleic acids, such as DNA and RNA. In plants, much of the nitrogen is used in chlorophyll molecules which are essential for photosynthesis and further growth.
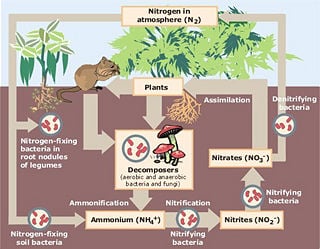
Processing, or fixation, is necessary to convert gaseous nitrogen into forms usable by living organisms. Some fixation occurs in lightning strikes, but most fixation is done by free-living or symbiotic bacteria. These bacteria have the nitrogenase enzyme that combines gaseous nitrogen with hydrogen to produce ammonia, which is then further converted by the bacteria to make its own organic compounds. Some nitrogen fixing bacteria, such as Rhizobium, live in the root nodules of legumes (such as peas or beans). Here they form a mutualistic relationship with the plant, producing ammonia in exchange for carbohydrates. Nutrient-poor soils can be planted with legumes to enrich them with nitrogen. A few other plants can form such symbioses.
Other plants get nitrogen from the soil by absorption at their roots in the form of either nitrate ions or ammonium ions. All nitrogen obtained by animals can be traced to the eating of plants at some stage of the food chain.
Ammonia
The source of ammonia is the decomposition of dead organic matter by bacteria called decomposers, which produce ammonium ions (NH4+). In well-oxygenated soil, these ions are then oxygenated first by nitrifying bacteria into nitrite (NO2-) and then into nitrate (NO3-). This two-step conversion of ammonium into nitrate is called nitrification.
Ammonia is highly toxic to fish life and the water discharge level of ammonia from wastewater treatment plants must often be closely monitored. To prevent loss of fish, nitrification prior to discharge is often desirable. Land application can be an attractive alternative to the mechanical aeration needed for nitrification. Ammonium ions readily bind to soils, especially to humic substances and clays. Nitrate and nitrite ions, due to their negative electric charge, bind less readily since there are less positively charged ion-exchange sites (mostly humic substances) in soil than negative. After rain or irrigation, leaching (the removal of soluble ions, such as nitrate and nitrite) into groundwater can occur. Elevated nitrate in groundwater is a concern for drinking water use because nitrate can interfere with blood-oxygen levels in infants and cause methemoglobinemia or blue-baby syndrome. Where groundwater recharges stream flow, nitrate-enriched groundwater can contribute to eutrophication, a process leading to high algal and blue-green bacterial populations and the death of aquatic life due to excessive demand for oxygen. While not directly toxic to fish life like ammonia, nitrate can have indirect effects on fish if it contributes to this eutrophication. Nitrogen has contributed to severe eutrophication problems in some water bodies. As of 2006, the application of nitrogen fertilizer is being increasingly controlled in Britain and the United States. This is occurring along the same lines as control of phosphorus fertilizer, restriction of which is normally considered essential to the recovery of eutrophied waterbodies.
During anaerobic (low oxygen) conditions, denitrification by bacteria occurs. This results in nitrates being converted to nitrogen gas and returned to the atmosphere.
Processes of the Nitrogen Cycle
Nitrogen Fixation
Conversion of N2 There are three ways to convert N2 (atmospheric nitrogen gas) into more chemically reactive forms: Biological fixation ; some symbiotic bacteria (most often associated with leguminous plants) and some free-living bacteria are able to fix nitrogen and assimilate it as organic nitrogen. Industrial N-fixation ; in the Haber-Bosch process, N2 is converted together with hydrogen gas (H2) into ammonia (NH3) fertilizer. An example of a mutualistic nitrogen fixing bacteria is the Rhizobium bacteria, which lives in plant root nodes. As well, there are free living bacteria, typically in the soil, such as the Azotobacter, that are responsible for nitrogen fixing. Combustion of fossil fuels ; automobile engines and thermal power plants, which release NOx. Additionally, the formation of NO from N2 and O2 due to photons and lightning, are important for atmospheric chemistry, but not for terrestrial or aquatic nitrogen turnover.
As a result of extensive cultivation of legumes (particularly soy, alfalfa, and clover), use of the Haber-Bosch process in the creation of chemical fertilizers and pollution emitted by vehicles and industrial plants, human beings have more than doubled the annual transfer of nitrogen into a biologically available form. This has occurred to the detriment of aquatic and wetland habitats through eutrophication.
Nitrification
The conversion of ammonia to nitrates is performed primarily by soil living bacteria. The primary stage of nitrification, the oxidation of ammonia (NH3) is performed by bacteria such as the Nitrosomonas species, which converts ammonia to nitrites (NO2-). Other bacterial species, such as the Nitrobacter, are responsible for the oxidation of the nitrites into nitrates (NO3-).
Ammonification
Nitrates are the form of nitrogen most commonly assimilated by plant species, which, in turn are consumed by heterotrophs for use in compounds such as amino and nucleic acids. The remains of heterotrophs will then be decomposed into nutrient rich organic material and bacteria or in some cases, fungi will convert the nitrates within the remains back into ammonia.
Denitrification
Denitrification is the reduction of nitrates back into the largely inert nitrogen gas (N2), completing the nitrogen cycle. This process is performed by bacterial species such as the Pseudomonas.
| Biogeochemical cycles |
|---|
| Carbon cycle - Hydrogen cycle - Nitrogen cycle |
| Oxygen cycle - Phosphorus cycle - Sulfur cycle - Water cycle |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.