Difference between revisions of "Mineral" - New World Encyclopedia
| Line 10: | Line 10: | ||
The [[quartz]], [[mica]]*, and [[feldspar]]* groups of minerals are common, while others have been found in only one or two locations worldwide. Over half the known mineral species are so rare that they have been found in only a handful of samples, and many are known from only one or two small grains. | The [[quartz]], [[mica]]*, and [[feldspar]]* groups of minerals are common, while others have been found in only one or two locations worldwide. Over half the known mineral species are so rare that they have been found in only a handful of samples, and many are known from only one or two small grains. | ||
| + | |||
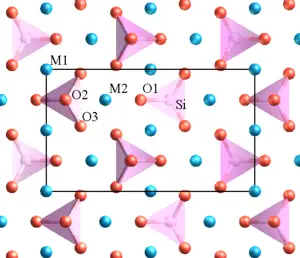
| + | [[Image:Atomic_structure_of_olivine_1.png|left|thumbnail|Atomic-scale crystal structure of olivine (magnesium iron silicate), as viewed along one axis (the ''a'' axis). Oxygen is shown in red, silicon in pink, and magnesium/iron in blue. A projection of the unit cell is indicated by the black rectangle.]] | ||
The '''crystal structure''' of a mineral is the orderly, geometric arrangement of [[atom]]s or [[ion]]s in the mineral's internal structure. There are 14 basic crystal lattice arrangements of atoms in three dimensions, and these are referred to as the 14 "Bravais lattices." Each of these lattices can be classified into one of the six "crystal systems." All currently recognized crystal structures fit into one Bravais lattice and one crystal system. Even when the mineral grains are too small to see or are irregularly shaped, the underlying crystal structure is always periodic and can be determined by a technique known as [[X ray|X-ray]] diffraction. | The '''crystal structure''' of a mineral is the orderly, geometric arrangement of [[atom]]s or [[ion]]s in the mineral's internal structure. There are 14 basic crystal lattice arrangements of atoms in three dimensions, and these are referred to as the 14 "Bravais lattices." Each of these lattices can be classified into one of the six "crystal systems." All currently recognized crystal structures fit into one Bravais lattice and one crystal system. Even when the mineral grains are too small to see or are irregularly shaped, the underlying crystal structure is always periodic and can be determined by a technique known as [[X ray|X-ray]] diffraction. | ||
| Line 15: | Line 17: | ||
The typical, outward appearance of a mineral is called the "crystal habit." Some crystal habits are distinctive of certain minerals, but in most cases, a mineral exhibits a variety of habits that are influenced by the growth conditions of the crystals. A mineral may show good crystal habit or form, or it may be massive, granular, or compact with only microscopically visible crystals. An inexperienced observer may be misled by a mineral's crystal habit, as the crystal system can be hidden or disguised. | The typical, outward appearance of a mineral is called the "crystal habit." Some crystal habits are distinctive of certain minerals, but in most cases, a mineral exhibits a variety of habits that are influenced by the growth conditions of the crystals. A mineral may show good crystal habit or form, or it may be massive, granular, or compact with only microscopically visible crystals. An inexperienced observer may be misled by a mineral's crystal habit, as the crystal system can be hidden or disguised. | ||
| − | Chemistry and crystal structure together define a mineral. When two or more minerals have the same chemical composition but differ in crystal structure, they are known as ''polymorphs''. For example, [[pyrite]] and [[marcasite]] are both iron sulfide, but their arrangement of atoms differs. Similarly, some minerals have different chemical compositions but the same crystal structure. For example, [[halite]] (made from sodium and [[chlorine]]), [[galena]] (made from [[lead]] and [[sulfur]]), and [[periclase]] (made from [[magnesium]] and [[oxygen]]) | + | Chemistry and crystal structure (and crystal habit) together define a mineral. The task of classification can range from being simple to complex. In some cases, knowledge of some properties may be sufficient for complete identification. In other cases, classification of a mineral may require more complex chemical or [[X-ray diffraction]] analyses. These methods can be costly and time-consuming, and may risk damaging the sample. |
| + | |||
| + | When two or more minerals have the same chemical composition but differ in crystal structure, they are known as ''polymorphs''. For example, [[pyrite]]* and [[marcasite]]* are both iron sulfide, but their arrangement of atoms differs. Similarly, some minerals have different chemical compositions but the same crystal structure. For example, [[halite]]* (made from sodium and [[chlorine]]), [[galena]]* (made from [[lead]] and [[sulfur]]), and [[periclase]]* (made from [[magnesium]] and [[oxygen]]) share the same cubic crystal structure. | ||
Crystal structure greatly influences a mineral's physical properties. For example, both [[diamond]] and [[graphite]] are pure [[carbon]]), but graphite is very soft, while diamond is the hardest of all known minerals. The reason for this difference is that the carbon atoms in graphite are arranged into sheets that can easily slide past one another, while the carbon atoms in diamond form a strong, interlocking three-dimensional network. | Crystal structure greatly influences a mineral's physical properties. For example, both [[diamond]] and [[graphite]] are pure [[carbon]]), but graphite is very soft, while diamond is the hardest of all known minerals. The reason for this difference is that the carbon atoms in graphite are arranged into sheets that can easily slide past one another, while the carbon atoms in diamond form a strong, interlocking three-dimensional network. | ||
| − | There are currently just over 4,000 known minerals, according to the [[International Mineralogical Association]], which is responsible for the approval and naming of newly discovered mineral species. | + | There are currently just over 4,000 known minerals, according to the [[International Mineralogical Association]]*, which is responsible for the approval and naming of newly discovered mineral species. |
=== Rocks === | === Rocks === | ||
| Line 25: | Line 29: | ||
A [[Rock (geology)|rock]] is an aggregate of two or more minerals, and it may also include organic remains. In some rocks, one mineral may be predominant. For example, [[limestone]] is a [[sedimentary]] rock composed almost entirely of the mineral [[calcite]]*. Other rocks contain many minerals, and the specific minerals in a rock can vary widely. | A [[Rock (geology)|rock]] is an aggregate of two or more minerals, and it may also include organic remains. In some rocks, one mineral may be predominant. For example, [[limestone]] is a [[sedimentary]] rock composed almost entirely of the mineral [[calcite]]*. Other rocks contain many minerals, and the specific minerals in a rock can vary widely. | ||
| − | + | == Physical properties == | |
| − | |||
| − | |||
| − | + | When identifying and classifying a mineral, the following physical properties are taken into consideration. | |
| − | |||
* ''Crystal structure and habit'', noted above. | * ''Crystal structure and habit'', noted above. | ||
| − | + | [[Image:Talc block.jpg|thumb|Talc is an extremely soft mineral, with a hardness of 1 on the Mohs scale.]] | |
| + | * The physical ''hardness'' (scratch resistance) of a mineral is usually measured on the Mohs scale, which ranges from 1 to 10. A mineral with a given Mohs hardness can scratch the surface of any mineral ranked lower in hardness. Certain minerals have been chosen to define the scale, as given below. | ||
::1- [[talc]]* | ::1- [[talc]]* | ||
::2- [[gypsum]]* | ::2- [[gypsum]]* | ||
| Line 44: | Line 46: | ||
::9- [[corundum]]* | ::9- [[corundum]]* | ||
::10- [[diamond]] | ::10- [[diamond]] | ||
| − | + | [[Image:GalenaFromKansas.jpg|thumb|220px|Galena (lead sulfide) has a metallic luster.]] | |
| − | * ''Luster'' | + | * ''Luster'' indicates the way the mineral's surface interacts with light. It can range from metallic to glassy (vitreous) to dull. |
::Metallic - high reflectivity like metal (e.g., galena) | ::Metallic - high reflectivity like metal (e.g., galena) | ||
::Sub-metallic - slightly less than metallic reflectivity (e.g., magnetite) | ::Sub-metallic - slightly less than metallic reflectivity (e.g., magnetite) | ||
| Line 60: | Line 62: | ||
* Other properties: [[fluorescence]]* (response to ultraviolet light), [[magnetism]], [[radioactivity]], [[tenacity]]* (response to mechanically induced changes of shape or form), and reactivity to dilute [[acid]]s. | * Other properties: [[fluorescence]]* (response to ultraviolet light), [[magnetism]], [[radioactivity]], [[tenacity]]* (response to mechanically induced changes of shape or form), and reactivity to dilute [[acid]]s. | ||
| − | + | == Chemical properties == | |
| − | + | The classification of minerals is also based on their chemical composition. Here they are categorized by their ''[[anion]]*'' groups. The list below, which follows the [[James Dwight Dana|Dana]]* classification system, is in approximate order of abundance of the minerals in the Earth's [[Crust (geology)|crust]]*. | |
| − | + | === Silicate class === | |
The largest group of minerals by far are the '''[[Silicate minerals|silicates]]*''' (most rocks are more than 95% silicates). They are composed largely of [[silicon]] and [[oxygen]], with the addition of ions such as [[aluminum]], [[magnesium]], [[iron]], and [[calcium]]. Some important rock-forming silicates include the [[feldspar]]s, [[quartz]], [[olivine]]*s, [[pyroxene]]*s, [[amphibole]]*s, [[garnet]]*s, and [[mica]]s. | The largest group of minerals by far are the '''[[Silicate minerals|silicates]]*''' (most rocks are more than 95% silicates). They are composed largely of [[silicon]] and [[oxygen]], with the addition of ions such as [[aluminum]], [[magnesium]], [[iron]], and [[calcium]]. Some important rock-forming silicates include the [[feldspar]]s, [[quartz]], [[olivine]]*s, [[pyroxene]]*s, [[amphibole]]*s, [[garnet]]*s, and [[mica]]s. | ||
| − | + | === Carbonate class === | |
The '''[[carbonate minerals]]*''' consist of those that contain the anion CO<sub>3</sub><sup>2-</sup>. They include [[calcite]]* and [[aragonite]]* (both calcium carbonate), [[dolomite]]* (magnesium/calcium carbonate), and [[siderite]]* (iron carbonate). Carbonates are commonly deposited in marine settings, when the shells of dead [[plankton|planktonic]] life settle and accumulate on the seafloor. Carbonates are also found in [[evaporite|evaporitic]]* settings (for example, the [[Great Salt Lake]]*, [[Utah]]) and in [[karst]]* regions, where the dissolution and reprecipitation of carbonates leads to the formation of [[cave]]s, [[stalactite]]*s and [[stalagmite]]*s. The carbonate class includes the [[nitrate]]* and [[borate]]* minerals. | The '''[[carbonate minerals]]*''' consist of those that contain the anion CO<sub>3</sub><sup>2-</sup>. They include [[calcite]]* and [[aragonite]]* (both calcium carbonate), [[dolomite]]* (magnesium/calcium carbonate), and [[siderite]]* (iron carbonate). Carbonates are commonly deposited in marine settings, when the shells of dead [[plankton|planktonic]] life settle and accumulate on the seafloor. Carbonates are also found in [[evaporite|evaporitic]]* settings (for example, the [[Great Salt Lake]]*, [[Utah]]) and in [[karst]]* regions, where the dissolution and reprecipitation of carbonates leads to the formation of [[cave]]s, [[stalactite]]*s and [[stalagmite]]*s. The carbonate class includes the [[nitrate]]* and [[borate]]* minerals. | ||
| − | + | === Sulfate class === | |
The '''[[sulfate]]*s''' contain the sulfate anion, SO<sub>4</sub><sup>2-</sup>. Sulfates commonly form in [[evaporite|evaporitic]]* settings, where highly saline waters slowly evaporate, allowing the formation of sulfates and halides at the water-sediment interface. Sulfates also occur in [[hydrothermal]]* vein systems as gangue minerals, along with [[sulfide]]* [[ore]]* minerals. Another occurrence is as secondary [[oxidation]]* products of original sulfide minerals. Common sulfates include [[anhydrite]]* (calcium sulfate), [[celestite]]* (strontium sulfate), [[barite]]* (barium sulfate), and [[gypsum]]* (hydrated calcium sulfate). The sulfate class also includes the [[chromate]]*, [[molybdate]]*, [[selenate]]*, [[sulfite]]*, [[tellurate]]*, and [[tungstate]]* minerals. | The '''[[sulfate]]*s''' contain the sulfate anion, SO<sub>4</sub><sup>2-</sup>. Sulfates commonly form in [[evaporite|evaporitic]]* settings, where highly saline waters slowly evaporate, allowing the formation of sulfates and halides at the water-sediment interface. Sulfates also occur in [[hydrothermal]]* vein systems as gangue minerals, along with [[sulfide]]* [[ore]]* minerals. Another occurrence is as secondary [[oxidation]]* products of original sulfide minerals. Common sulfates include [[anhydrite]]* (calcium sulfate), [[celestite]]* (strontium sulfate), [[barite]]* (barium sulfate), and [[gypsum]]* (hydrated calcium sulfate). The sulfate class also includes the [[chromate]]*, [[molybdate]]*, [[selenate]]*, [[sulfite]]*, [[tellurate]]*, and [[tungstate]]* minerals. | ||
| − | + | === Halide class === | |
The '''[[halide]]*s''' are a group of minerals that form [[salt]]s such as [[fluorite]]* (calcium fluoride), [[halite]]* (sodium chloride), [[sylvite]]* (potassium chloride), and [[sal ammoniac]]* (ammonium chloride). Like the sulfates, halides are commonly found in evaporitic settings such as [[Playa|playa lake]]s (lakebeds that are usually dry) and landlocked seas, such as the [[Dead Sea]]* and Great Salt Lake. The halide class includes the [[fluoride]]*, [[chloride]]*, and [[iodide]]* minerals. | The '''[[halide]]*s''' are a group of minerals that form [[salt]]s such as [[fluorite]]* (calcium fluoride), [[halite]]* (sodium chloride), [[sylvite]]* (potassium chloride), and [[sal ammoniac]]* (ammonium chloride). Like the sulfates, halides are commonly found in evaporitic settings such as [[Playa|playa lake]]s (lakebeds that are usually dry) and landlocked seas, such as the [[Dead Sea]]* and Great Salt Lake. The halide class includes the [[fluoride]]*, [[chloride]]*, and [[iodide]]* minerals. | ||
| − | + | === Oxide class === | |
| − | '''[[Oxide]]s''' are extremely important in [[mining]] as they form many of the ores from which valuable metals | + | '''[[Oxide]]s*''' are extremely important in [[mining]], as they form many of the ores from which valuable metals are extracted. They commonly occur as precipitates close to the Earth's surface, [[oxidation]] products of other minerals in the near-surface [[weathering]]* zone, and as accessory minerals in igneous rocks of the crust and [[Mantle (geology)|mantle]]. Common oxides include [[hematite]]* (iron oxide), [[magnetite]]* (iron oxide), [[chromite]]* (chromium oxide), [[spinel]]* (magnesium aluminium oxide, a common component of the mantle), [[rutile]]* (titanium dioxide), and [[ice]]* (hydrogen oxide). The oxide class includes the [[hydroxide]]* minerals. |
| − | + | === Sulfide class === | |
| − | Many '''[[sulfide]]s''' are economically important as metal ores. | + | Many '''[[sulfide]]s*''' are economically important as metal ores. Common sulfides include [[pyrite]]* (iron sulfide, also known as ''fools' gold''), [[chalcopyrite]]* (copper iron sulfide), [[pentlandite]]* (nickel iron sulfide), and galena (lead sulfide). The sulfide class also includes the [[selenide]]*s, [[telluride]]*s, [[arsenide]]*s, [[antimonide]]*s, bismuthinides, and sulfo salts (containing sulfide and a second anion such as arsenide). |
| − | + | === Phosphate class === | |
| − | The '''[[phosphate mineral]]''' group | + | The '''[[phosphate mineral]]*''' group includes any mineral in which the anion takes the tetrahedral form AO<sub>4</sub><sup>-n</sup>, where A can be [[phosphorus]], [[antimony]], [[arsenic]] or [[vanadium]]. The most common phosphate by far is [[apatite]]*, which is an important [[biology|biological]] mineral found in the teeth and bones of many animals. |
| − | + | === Element class === | |
| − | The | + | The '''element''' group includes metals, metalloids, and nonmetals. Minerals in this group include gold, silver, copper, antimony, bismuth, graphite, and sulfur. This group also includes naturally occurring alloys (such as [[electrum]]*, an alloy of gold and silver), phosphides, silicides, nitrides, and carbides (which are found naturally in a few, rare meteorites). |
==See also== | ==See also== | ||
| − | * [[List | + | * [[List of minerals]] (with associated articles) |
| − | * [[List of minerals (complete)| | + | * [[List of minerals (complete)|Comprehensive list of minerals]] |
* [[Industrial minerals]] | * [[Industrial minerals]] | ||
* [[Mineral water]], water containing minerals or other dissolved substances that alter its taste or give it therapeutic value | * [[Mineral water]], water containing minerals or other dissolved substances that alter its taste or give it therapeutic value | ||
| − | |||
* [[Mining]] | * [[Mining]] | ||
| − | |||
* [[Quarry]] | * [[Quarry]] | ||
* [[Dietary mineral]] | * [[Dietary mineral]] | ||
Revision as of 19:56, 6 June 2006
- For other uses, see Mineral (disambiguation).
Minerals are natural compounds formed through geological processes. The term "mineral" encompasses not only the material's chemical composition, but also the mineral's structure. Minerals range in composition from pure elements and simple salts to very complex silicates with thousands of known forms (organic compounds are usually excluded). The study of minerals is called mineralogy.

Mineral definition and classification
A mineral is defined as a naturally occurring, inorganic solid with a definite chemical composition and crystalline structure. Mineral-like substances that do not strictly meet this definition are sometimes classified as mineraloids. Other naturally occurring substances are called nonminerals. "Industrial minerals" is a market term and refers to commercially valuable, mined materials.
Minerals that are closely related in composition and structure are grouped together. For example, the feldspar group of minerals, which make up as much as 60% of the Earth's crust, can be subdivided into potassium feldspars and plagioclase feldspars. The latter subgroup consists of a continuous series of minerals, from sodium-rich albite (NaAlSi3O8) to calcium-rich anorthite (CaAl2Si2O8), with four recognized intermediate compositions.
The quartz, mica, and feldspar groups of minerals are common, while others have been found in only one or two locations worldwide. Over half the known mineral species are so rare that they have been found in only a handful of samples, and many are known from only one or two small grains.
The crystal structure of a mineral is the orderly, geometric arrangement of atoms or ions in the mineral's internal structure. There are 14 basic crystal lattice arrangements of atoms in three dimensions, and these are referred to as the 14 "Bravais lattices." Each of these lattices can be classified into one of the six "crystal systems." All currently recognized crystal structures fit into one Bravais lattice and one crystal system. Even when the mineral grains are too small to see or are irregularly shaped, the underlying crystal structure is always periodic and can be determined by a technique known as X-ray diffraction.
The typical, outward appearance of a mineral is called the "crystal habit." Some crystal habits are distinctive of certain minerals, but in most cases, a mineral exhibits a variety of habits that are influenced by the growth conditions of the crystals. A mineral may show good crystal habit or form, or it may be massive, granular, or compact with only microscopically visible crystals. An inexperienced observer may be misled by a mineral's crystal habit, as the crystal system can be hidden or disguised.
Chemistry and crystal structure (and crystal habit) together define a mineral. The task of classification can range from being simple to complex. In some cases, knowledge of some properties may be sufficient for complete identification. In other cases, classification of a mineral may require more complex chemical or X-ray diffraction analyses. These methods can be costly and time-consuming, and may risk damaging the sample.
When two or more minerals have the same chemical composition but differ in crystal structure, they are known as polymorphs. For example, pyrite and marcasite are both iron sulfide, but their arrangement of atoms differs. Similarly, some minerals have different chemical compositions but the same crystal structure. For example, halite (made from sodium and chlorine), galena (made from lead and sulfur), and periclase (made from magnesium and oxygen) share the same cubic crystal structure.
Crystal structure greatly influences a mineral's physical properties. For example, both diamond and graphite are pure carbon), but graphite is very soft, while diamond is the hardest of all known minerals. The reason for this difference is that the carbon atoms in graphite are arranged into sheets that can easily slide past one another, while the carbon atoms in diamond form a strong, interlocking three-dimensional network.
There are currently just over 4,000 known minerals, according to the International Mineralogical Association, which is responsible for the approval and naming of newly discovered mineral species.
Rocks
A rock is an aggregate of two or more minerals, and it may also include organic remains. In some rocks, one mineral may be predominant. For example, limestone is a sedimentary rock composed almost entirely of the mineral calcite. Other rocks contain many minerals, and the specific minerals in a rock can vary widely.
Physical properties
When identifying and classifying a mineral, the following physical properties are taken into consideration.
- Crystal structure and habit, noted above.
- The physical hardness (scratch resistance) of a mineral is usually measured on the Mohs scale, which ranges from 1 to 10. A mineral with a given Mohs hardness can scratch the surface of any mineral ranked lower in hardness. Certain minerals have been chosen to define the scale, as given below.
- Luster indicates the way the mineral's surface interacts with light. It can range from metallic to glassy (vitreous) to dull.
- Metallic - high reflectivity like metal (e.g., galena)
- Sub-metallic - slightly less than metallic reflectivity (e.g., magnetite)
- Vitreous - the luster of broken glass (e.g., quartz)
- Pearly - very soft light shown by some layer silicates (e.g., talc)
- Silky - soft light shown by fibrous materials (e.g., gypsum)
- Dull/earthy - shown by finely crystallized minerals (e.g., kidney ore variety of hematite)
- Color indicates the mineral's appearance as observed by the naked eye. Technically, it means the color of reflected light, if the mineral is opaque, or the color of transmitted light, if the mineral is translucent.
- Streak refers to the color of the powder produced from a mineral after it has been rubbed on an unglazed porcelain streak plate.
- Cleavage describes the way a mineral may split apart along various planes. In thin section, cleavage is visible as thin, parallel lines across a mineral.
- Fracture describes how a mineral breaks when broken contrary to its natural cleavage planes. For example, a chonchoidal fracture is a smooth fracture with concentric ridges of the type shown by glass.
- Specific gravity corresponds to the density of the material compared to that of water. Most minerals, including all rock-forming minerals, have a specific gravity of 2.5–3.5. Some, however, are noticeably more or less dense. For example, the specific gravity of several sulphide minerals is higher than that of the common, rock-forming minerals.
- Other properties: fluorescence (response to ultraviolet light), magnetism, radioactivity, tenacity (response to mechanically induced changes of shape or form), and reactivity to dilute acids.
Chemical properties
The classification of minerals is also based on their chemical composition. Here they are categorized by their anion groups. The list below, which follows the Dana classification system, is in approximate order of abundance of the minerals in the Earth's crust.
Silicate class
The largest group of minerals by far are the silicates (most rocks are more than 95% silicates). They are composed largely of silicon and oxygen, with the addition of ions such as aluminum, magnesium, iron, and calcium. Some important rock-forming silicates include the feldspars, quartz, olivines, pyroxenes, amphiboles, garnets, and micas.
Carbonate class
The carbonate minerals consist of those that contain the anion CO32-. They include calcite and aragonite (both calcium carbonate), dolomite (magnesium/calcium carbonate), and siderite (iron carbonate). Carbonates are commonly deposited in marine settings, when the shells of dead planktonic life settle and accumulate on the seafloor. Carbonates are also found in evaporitic settings (for example, the Great Salt Lake, Utah) and in karst regions, where the dissolution and reprecipitation of carbonates leads to the formation of caves, stalactites and stalagmites. The carbonate class includes the nitrate and borate minerals.
Sulfate class
The sulfates contain the sulfate anion, SO42-. Sulfates commonly form in evaporitic settings, where highly saline waters slowly evaporate, allowing the formation of sulfates and halides at the water-sediment interface. Sulfates also occur in hydrothermal vein systems as gangue minerals, along with sulfide ore minerals. Another occurrence is as secondary oxidation products of original sulfide minerals. Common sulfates include anhydrite (calcium sulfate), celestite (strontium sulfate), barite (barium sulfate), and gypsum (hydrated calcium sulfate). The sulfate class also includes the chromate, molybdate, selenate, sulfite, tellurate, and tungstate minerals.
Halide class
The halides are a group of minerals that form salts such as fluorite (calcium fluoride), halite (sodium chloride), sylvite (potassium chloride), and sal ammoniac (ammonium chloride). Like the sulfates, halides are commonly found in evaporitic settings such as playa lakes (lakebeds that are usually dry) and landlocked seas, such as the Dead Sea and Great Salt Lake. The halide class includes the fluoride, chloride, and iodide minerals.
Oxide class
Oxides* are extremely important in mining, as they form many of the ores from which valuable metals are extracted. They commonly occur as precipitates close to the Earth's surface, oxidation products of other minerals in the near-surface weathering zone, and as accessory minerals in igneous rocks of the crust and mantle. Common oxides include hematite (iron oxide), magnetite (iron oxide), chromite (chromium oxide), spinel (magnesium aluminium oxide, a common component of the mantle), rutile (titanium dioxide), and ice (hydrogen oxide). The oxide class includes the hydroxide minerals.
Sulfide class
Many sulfides* are economically important as metal ores. Common sulfides include pyrite (iron sulfide, also known as fools' gold), chalcopyrite (copper iron sulfide), pentlandite (nickel iron sulfide), and galena (lead sulfide). The sulfide class also includes the selenides, tellurides, arsenides, antimonides, bismuthinides, and sulfo salts (containing sulfide and a second anion such as arsenide).
Phosphate class
The phosphate mineral group includes any mineral in which the anion takes the tetrahedral form AO4-n, where A can be phosphorus, antimony, arsenic or vanadium. The most common phosphate by far is apatite, which is an important biological mineral found in the teeth and bones of many animals.
Element class
The element group includes metals, metalloids, and nonmetals. Minerals in this group include gold, silver, copper, antimony, bismuth, graphite, and sulfur. This group also includes naturally occurring alloys (such as electrum, an alloy of gold and silver), phosphides, silicides, nitrides, and carbides (which are found naturally in a few, rare meteorites).
See also
- List of minerals (with associated articles)
- Comprehensive list of minerals
- Industrial minerals
- Mineral water, water containing minerals or other dissolved substances that alter its taste or give it therapeutic value
- Mining
- Quarry
- Dietary mineral
External links
- Mineral gallery
- Minerals.net
- Information on minerals
- mindat.org mineral database
- Webmineral.com
- a directory of on-line databases related to mineralogy and crystallography
ReferencesISBN links support NWE through referral fees
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.