Ibuprofen
| |
| |
Ibuprofen
| |
| Systematic name | |
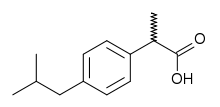
| IUPAC name 2-[4-(2-methylpropyl)phenyl]propanoic acid | |
| Identifiers | |
| CAS number | 15687-27-1 |
| ATC code | M01AE01 |
| PubChem | 3672 |
| DrugBank | APRD00372 |
| Chemical data | |
| Formula | C13H18O2 |
| Mol. weight | 206.3 g/mol |
| Physical data | |
| Melt. point | 76°C (169°F) |
| Pharmacokinetic data | |
| Bioavailability | 49–73% |
| Protein binding | 99% |
| Metabolism | Hepatic |
| Half life | 1.8–2 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | ? |
| Legal status | ? |
| Routes | Oral, rectal and topical |
Ibuprofen (INN) (IPA: [ˈaɪbjupɹofɛn]) (from the earlier nomenclature iso-butyl-propanoic-phenolic acid) is a non-steroidal anti-inflammatory drug (NSAID) originally marketed as Nurofen and since under various trademarks including Act-3, Advil, Brufen, Dorival, Herron Blue, Panafen, Motrin, Nuprin and Ipren or Ibumetin (Sweden), Ibuprom (Poland), IbuHEXAL, Moment (Italy), Ibux (Norway), Íbúfen (Iceland), Ibalgin (Czech Republic). It is used for relief of symptoms of arthritis, primary dysmenorrhoea, fever, and as an analgesic, especially where there is an inflammatory component. Ibuprofen has no antiplatelet effect.
History
Ibuprofen was developed by the research arm of Boots Group during the 1960s.
Clinical use
Low doses of ibuprofen (200 mg, and sometimes 400 mg) are available over the counter (OTC) in most countries. Ibuprofen has a dose-dependent duration of action of approximately 4–8 hours, which is longer than suggested by its short half-life. The recommended dose varies with body mass and indication. Generally, the oral dose is 200–400 mg (5–10 mg/kg in children) every 4–6 hours, adding up to a usual daily dose of 800–1200 mg. 1200 mg is considered the maximum daily dose for over-the-counter use, though under medical direction, a maximum daily dose of 3200 mg may sometimes be used in increments of 600—800 mg.
Off-Label and investigational use
- As with other NSAIDs, ibuprofen may be useful in the treatment of severe orthostatic hypotension.[1]
- In some studies, ibuprofen showed superior results compared to placebo in the prophylaxis of Alzheimer's disease, when given in low doses over a long time.[2] Further studies are needed to confirm the results before ibuprofen can be recommended for this indication.
- Ibuprofen has been associated with a lower risk of Parkinson's disease, and may delay or prevent Parkinson's disease. Aspirin, other NSAIDs, and paracetamol had no effect on the risk for Parkinson's.[3] Further research is warranted before recommending ibuprofen for this use.
Ibuprofen lysine
In Europe, Australia, and New Zealand, ibuprofen lysine (ibuprofenlysinat, the lysine salt of ibuprofen) is licensed for treatment of the same conditions as ibuprofen. Ibuprofen lysine has been shown to have a more rapid onset of action compared to base ibuprofen.[4]
Mechanism of action
Ibuprofen is an NSAID which is believed to work through inhibition of cyclooxygenase (COX), thus inhibiting prostaglandin synthesis. There are at least 2 variants of cyclooxygenase (COX-1 and COX-2). Ibuprofen inhibits both COX-1 and COX-2. It appears that its analgesic, antipyretic, and anti-inflammatory activity are achieved principally through COX-2 inhibition; whereas COX-1 inhibition is responsible for its unwanted effects on platelet aggregation and the GI mucosa.
Side effects
Ibuprofen appears to have the lowest incidence of gastrointestinal adverse drug reactions (ADRs) of all the non-selective NSAIDs. However, this only holds true at lower doses of ibuprofen, so over-the-counter preparations of ibuprofen are generally labeled to advise a maximum daily dose of 1,200 mg.
Reported adverse drug reactions
Common adverse effects include: nausea, dyspepsia, gastrointestinal ulceration/bleeding, raised liver enzymes, diarrhea, headache, dizziness, priapism, salt and fluid retention, and hypertension.[5]
Infrequent adverse effects include: oesophageal ulceration, heart failure, hyperkalaemia, renal impairment, confusion, bronchospasm, rash.[5]
Very infrequent adverse effects include Stevens-Johnson syndrome.
Photosensitivity
As with other NSAIDs, ibuprofen has been reported to be a photosensitising agent.[6][7] However, this only rarely occurs with ibuprofen and it is considered to be a very weak photosensitising agent when compared with other members of the 2-arylpropionic acids. This is because the ibuprofen molecule contains only a single phenyl moiety and no bond conjugation, resulting in a very weak chromophore system and a very weak absorption spectrum which does not reach into the solar spectrum.
Cardiovascular risk
Along with several other NSAIDs, ibuprofen has been implicated in elevating the risk of myocardial infarction, particularly among those chronically using high doses.[8]
Pregnancy risks
Two studies have found an increased risk of miscarriage with the use of NSAIDs such as ibuprofen early in pregnancy; however, several other studies did not find this association. There are also concerns that drugs such as ibuprofen may interfere with implantation of the early fetus, although a clear risk has not been established.
When ibuprofen is used as directed in the first and second trimester of pregnancy, it is not associated with an increased risk for birth defects. However, ibuprofen is generally not the pain reliever of choice during pregnancy because there are concerns with the use of ibuprofen during the third trimester.
DOMS
A study has shown that athletes who take ibuprofen to treat delayed onset muscle soreness (DOMS) show less muscle gain than athletes who did not.[citation needed]
Stereochemistry
Ibuprofen, like other 2-arylpropionate derivatives (including ketoprofen, flurbiprofen, naproxen, etc), contains a chiral carbon in the α-position of the propionate moiety. As such there are two possible enantiomers of ibuprofen with the potential for different biological effects and metabolism for each enantiomer.
Indeed it was found that (S)-(+)-ibuprofen (dexibuprofen) was the active form both in vitro and in vivo.
It was logical, then, that there was the potential for improving the selectivity and potency of ibuprofen formulations by marketing ibuprofen as a single-enantiomer product (as occurs with naproxen, another NSAID.).
Further in vivo testing, however, revealed the existence of an isomerase which converted (R)-ibuprofen to the active (S)-enantiomer(citation needed). Thus, due to the expense and futility that might be involved in marketing the single-enantiomer, most ibuprofen formulations currently marketed are racemic mixtures. A notable exception to this is Seractiv (Nordic Drugs).
Human toxicology
Ibuprofen overdose has become common since it was licensed for over-the-counter use. There are many overdose experiences reported in the medical literature.[9] Human response in cases of overdose ranges from absence of symptoms to fatal outcome in spite of intensive care treatment. Most symptoms are an excess of the pharmacological action of ibuprofen and include abdominal pain, nausea, vomiting, drowsiness, dizziness, headache, tinnitus, and nystagmus. Rarely more severe symptoms such as gastrointestinal bleeding, seizures, metabolic acidosis, hyperkalaemia, hypotension, bradycardia, tachycardia, atrial fibrillation, coma, hepatic dysfunction, acute renal failure, cyanosis, respiratory depression, and cardiac arrest have been reported.[10] The severity of symptoms varies with the ingested dose and the time elapsed, however, individual sensitivity also plays an important role. Generally, the symptoms observed with an overdose of ibuprofen are similar to the symptoms caused by overdoses of other NSAIDs.
There is little correlation between severity of symptoms and measured ibuprofen plasma levels. Toxic effects are unlikely at doses below 100 mg/kg but can be severe above 400 mg/kg;[11] however, large doses do not indicate that the clinical course is likely to be lethal.[12] It is not possible to determine a precise lethal dose, as this may vary with age, weight, and concomitant diseases of the individual patient.
Therapy is largely symptomatic. In cases presenting early, gastric decontamination is recommended. This is achieved using activated charcoal; charcoal absorbs the drug before it can enter the systemic circulation. Gastric lavage is now rarely used, but can be considered if the amount ingested is potentially life threatening and it can be performed within 60 minutes of ingestion. Emesis is not recommended.[13] The majority of ibuprofen ingestions produce only mild effects and the management of overdose is straightforward. Standard measures to maintain normal urine output should be instituted and renal function monitored.[11] Since ibuprofen has acidic properties and is also excreted in the urine, forced alkaline diuresis is theoretically beneficial. However, due to the fact ibuprofen is highly protein bound in the blood, there is minimal renal excretion of unchanged drug. Forced alkaline diuresis is therefore of limited benefit.[14] Symptomatic therapy for hypotension, GI bleeding, acidosis, and renal toxicity may be indicated. Occasionally, close monitoring in an intensive care unit for several days is necessary. If a patient survives the acute intoxication, he/she will usually experience no late sequelae.
Availability
Template:Globalize/Eng
Ibuprofen was made available under prescription in the United Kingdom in 1969, and in the United States in 1974. In the years since, the good tolerability profile along with extensive experience in the community (otherwise known as Phase IV trials), has resulted in the rescheduling of small packs of ibuprofen to allow availability over-the-counter in pharmacies worldwide, and indeed in supermarkets and other general retailers. For some time, there has been a limit on the amount that can be bought over the counter in a single transaction in the UK; this being 2 packs of 16 x 200 mg or 1 pack of 8 or 16 x 400 mg, the latter being far less common for over-the-counter sales. In the United States, the Food and Drug Administration approved it for over-the-counter use in 1984. The wider availability has meant that ibuprofen is now almost as commonly used as aspirin and paracetamol (acetaminophen).[citation needed] In other countries, such as Spain, higher dosages of 600 mg are available. Mexico's doses go up to 800mg per pill.
See also
ReferencesISBN links support NWE through referral fees
- ↑ Zawada E (1982). Renal consequences of nonsteroidal antiinflammatory drugs.. Postgrad Med 71 (5): 223-30. PMID 7041104.
- ↑ Townsend K, Praticò D (2005). Novel therapeutic opportunities for Alzheimer's disease: focus on nonsteroidal anti-inflammatory drugs.. FASEB J 19 (12): 1592-601. PMID 16195368.
- ↑ Chen H, Jacobs E, Schwarzschild M, McCullough M, Calle E, Thun M, Ascherio A (2005). Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease.. Ann Neurol 58 (6): 963-7. PMID 16240369.
- ↑ Geisslinger G, Dietzel K, Bezler H, Nuernberg B, Brune K (1989). Therapeutically relevant differences in the pharmacokinetical and pharmaceutical behavior of ibuprofen lysinate as compared to ibuprofen acid.. Int J Clin Pharmacol Ther Toxicol 27 (7): 324-8. PMID 2777420.
- ↑ 5.0 5.1 (2004) in Rossi S: Australian Medicines Handbook, 2004, Australian Medicines Handbook. ISBN 0-9578521-4-2.
- ↑ Bergner T, Przybilla B. Photosensitization caused by ibuprofen. J Am Acad Dermatol 1992;26(1):114-6. PMID 1531054
- ↑ Thomson Healthcare. USP DI Advice for the Patient: Anti-inflammatory Drugs, Nonsteroidal (Systemic) [monograph on the internet]. Bethesda (MD): U.S. National Library of Medicine; c2006 [updated 2006 Jul 28; cited 2006 Aug 5]. Available from: http://www.nlm.nih.gov/medlineplus/druginfo/uspdi/202743.html
- ↑ Hippisley-Cox J, Coupland C (2005). Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis.. BMJ 330 (7504): 1366. PMID 15947398.
- ↑ McElwee NE, Veltri JC, Bradford DC, Rollins DE. (1990). A prospective, population-based study of acute ibuprofen overdose: complications are rare and routine serum levels not warranted.. Ann Emerg Med 19 (6): 657-62. PMID 2188537.
- ↑ Vale JA, Meredith TJ. (1986). Acute poisoning due to non-steroidal anti-inflammatory drugs. Clinical features and management.. Med Toxicol 1 (1): 12-31. PMID 3537613.
- ↑ 11.0 11.1 Volans G, Hartley V, McCrea S, Monaghan J. (2003). Non-opioid analgesic poisoning. Clinical Medicine 3 (2): 119-23. PMID 12737366.
- ↑ Seifert SA, Bronstein AC, McGuire T. (2000). Massive ibuprofen ingestion with survival.. J Toxicol Clin Toxicol 38 (1): 55-7. PMID 10696926.
- ↑ (2004). Position paper: Ipecac syrup.. J Toxicol Clin Toxicol 42 (2): 133-43. PMID 15214617.
- ↑ Hall AH, Smolinske SC, Conrad FL, Wruk KM, Kulig KW, Dwelle TL, Rumack BH. (1986). Ibuprofen overdose: 126 cases.. Ann Emerg Med 15 (11): 1308–13. PMID 3777588.
External links
- U.S. National Library of Medicine: MedlinePlus Drug Information: Ibuprofen
- University of Bristol chemistry department page on Ibuprofen
- Ball and stick model
| |||||||||||||||||||||||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.