Difference between revisions of "Glutamic acid" - New World Encyclopedia
({{Contracted}}) |
Rick Swarts (talk | contribs) |
||
| Line 11: | Line 11: | ||
'''Glutamic acid''' (Glu, E), also referred to as '''glutamate''' (the [[anion]]), is one of the 20 [[proteinogenic]] [[amino acid]]s. It is not among the [[essential amino acid]]s. | '''Glutamic acid''' (Glu, E), also referred to as '''glutamate''' (the [[anion]]), is one of the 20 [[proteinogenic]] [[amino acid]]s. It is not among the [[essential amino acid]]s. | ||
| + | |||
| + | '''Aspartic acid''', also called '''asparaginic acid''' and '''alpha-aminosuccinic acid''' is an acidic, α-[[amino acid]] that is found in many [[protein]]s, is common in young [[sugar cane]] and [[sugar beet]]s, and is closely related to the amino acid [[asparagine]]. Along with [[glutamic acid]], it is classified as an acidic amino acid. | ||
| + | |||
| + | In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 [[amino acid#standard amino acid|standard amino acids]] required for normal functioning. However, it is considered to be [[amino acid#essential amino acid|non-essential]] since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. | ||
| + | |||
| + | Aspartic acid is pervasive in biosynthesis and is the precursor to several amino acids. Aspartic acid is a metabolite in the [[urea cycle]] and participates in [[gluconeogenesis]]. It also acts as a [[neurotransmitter]]. The non-[[carbohydrate]], non-nutritive artificial sweetener and flavor enhancer [[aspartame]] (aspartyl-phenylalanine-1-methyl ester) is synthesized from aspartic acid and the essential amino acid, [[phenylalanine]]. | ||
| + | |||
| + | The discovery, manufacture, and use of the sweetener aspartame, which is now found in many products, represents an aspect of human creativity, addressing a human desire for sweet things while trying to avoid the deleterious health consequences traced to overconsumption of sugar. However, human creativity can be for good or bad, and some health risks have been alleged for aspartame. | ||
| + | |||
| + | Aspartic acid's three letter code is ASP, its one letter code is D, its codons are GAU and GAC, and its systematic name is 2-Aminobutanedioic acid (IUPAC-IUB 1983). | ||
| + | |||
| + | A three-letter designation for either [[glutamine|Gln]] or Glu is '''Glx'''—this is often used in cases in which peptide sequencing reactions may convert glutamine to glutamate (or vice versa), leaving the original identity of the amino acid in doubt. | ||
| + | The one-letter abbreviation is E for glutamic acid and Q for glutamine. | ||
| + | |||
| + | Glutamic acid Glud E d 2-Aminopentanedioic acid HOOC-[CH2]2-CH(NH2)-COOH | ||
==Structure== | ==Structure== | ||
| + | In [[biochemistry]], the term [[amino acid]] is frequently used to refer specifically to ''alpha amino acids'': those amino acids in which the amino and carboxylate groups are attached to the same [[carbon]], the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is: | ||
| + | |||
| + | ''R'' | ||
| + | | | ||
| + | H<sub>2</sub>N-C-COOH | ||
| + | | | ||
| + | H | ||
| + | where ''R'' represents a ''side chain'' specific to each amino acid. | ||
| + | |||
| + | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In aspartic acid, only the L-stereoisomer is involved in protein synthesis. | ||
| + | |||
| + | Aspartic acids chemical formula is HOOC-CH(NH<sub>2</sub>)-CH<sub>2</sub>-COOH, or more generally C<sub>4</sub>H<sub>7</sub>NO<sub>4</sub>. | ||
| + | |||
| + | Aspartic acid behaves similarly to glutamic acid. It carries a hydrophilic acidic group with strong negative charge. Aspartic acid usually is located on the outer surface of the protein, making it water-soluble. It binds to positively-charged molecules and ions, often used in [[enzyme]]s to fix the metal ion. | ||
| + | |||
| + | |||
As its name indicates, it is [[acid]]ic, with a [[carboxylic acid]] component to its [[side chain]]. Generally either the [[amino group]] will be [[protonate|protonated]] or one or both of the [[carboxylic group]]s will be [[deprotonation|deprotonated]]. At neutral [[pH]] all three groups are ionized and the species has a charge of -1. The pKa value for Glutamic acid is 4.1. This means that at pH below this value it will be protonated (COOH) and at pH above this value it will be deprotonated (COO-) | As its name indicates, it is [[acid]]ic, with a [[carboxylic acid]] component to its [[side chain]]. Generally either the [[amino group]] will be [[protonate|protonated]] or one or both of the [[carboxylic group]]s will be [[deprotonation|deprotonated]]. At neutral [[pH]] all three groups are ionized and the species has a charge of -1. The pKa value for Glutamic acid is 4.1. This means that at pH below this value it will be protonated (COOH) and at pH above this value it will be deprotonated (COO-) | ||
| − | + | ||
| − | |||
==Synthesis== | ==Synthesis== | ||
| Line 67: | Line 97: | ||
===As a neurotransmitter=== | ===As a neurotransmitter=== | ||
Glutamate is the most abundant fast excitatory [[neurotransmitter]] in the mammalian [[nervous system]]. At [[synapses|chemical synapses]], glutamate is stored in vesicles. [[Nerve impulses]] trigger release of glutamate from the pre-synaptic cell. In the opposing post-synaptic cell, [[glutamate receptors]], such as the [[NMDA receptor]], bind glutamate and are activated. Because of its role in [[synaptic plasticity]], it is believed that glutamic acid is involved in cognitive functions like [[learning]] and [[memory]] in the brain. | Glutamate is the most abundant fast excitatory [[neurotransmitter]] in the mammalian [[nervous system]]. At [[synapses|chemical synapses]], glutamate is stored in vesicles. [[Nerve impulses]] trigger release of glutamate from the pre-synaptic cell. In the opposing post-synaptic cell, [[glutamate receptors]], such as the [[NMDA receptor]], bind glutamate and are activated. Because of its role in [[synaptic plasticity]], it is believed that glutamic acid is involved in cognitive functions like [[learning]] and [[memory]] in the brain. | ||
| + | |||
| + | Note: As a [[neurotransmitter]], aspartate (the conjugate base of aspartic acid) stimulates [[NMDA receptor]]s, though not as strongly as the amino acid neurotransmitter [[glutamic acid|glutamate]] does (Chen et al. 2005). It serves as an excitatory neurotransmitter in the brain and is an excitotoxin. | ||
| + | |||
[[Glutamate transporter]]s{{ref_N|3|a}} are found in [[neuron]]al and [[glia]]l membranes. They rapidly remove glutamate from the [[extracellular]] space. In brain injury or disease, they can work in reverse and excess glutamate can accumulate outside cells. This process causes calcium ions to enter cells via [[NMDA receptor]] channels, leading to neuronal damage and eventual cell death, and is called [[excitotoxicity]]. The mechanisms of [[apoptosis|cell death]] include: | [[Glutamate transporter]]s{{ref_N|3|a}} are found in [[neuron]]al and [[glia]]l membranes. They rapidly remove glutamate from the [[extracellular]] space. In brain injury or disease, they can work in reverse and excess glutamate can accumulate outside cells. This process causes calcium ions to enter cells via [[NMDA receptor]] channels, leading to neuronal damage and eventual cell death, and is called [[excitotoxicity]]. The mechanisms of [[apoptosis|cell death]] include: | ||
| Line 110: | Line 143: | ||
#{{note_N|7|}} {{cite journal | author=Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE | title=Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo | journal=Journal of Neuroscience | volume=27 | issue=1 | year=2007 | pages=111-123 | id=PMID 17202478}} | #{{note_N|7|}} {{cite journal | author=Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE | title=Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo | journal=Journal of Neuroscience | volume=27 | issue=1 | year=2007 | pages=111-123 | id=PMID 17202478}} | ||
#{{note_N|8|}} {{cite journal | author=Zheng Xi, Baker DA, Shen H, Carson DS, Kalivas PW | title=Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens | journal=Journal of Pharmacology and Experimental Therapeutics | volume=300 | issue=1 | year=2002 | pages=162-171 | id=PMID 11752112}} | #{{note_N|8|}} {{cite journal | author=Zheng Xi, Baker DA, Shen H, Carson DS, Kalivas PW | title=Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens | journal=Journal of Pharmacology and Experimental Therapeutics | volume=300 | issue=1 | year=2002 | pages=162-171 | id=PMID 11752112}} | ||
| + | |||
| + | |||
| + | * Chen, P. E., M. T. Geballe, P. J. Stansfeld, A. R. Johnston, H. Yuan, A. L. Jacob, J. P. Snyder, S. F. Traynelis, and D. J. A. Wyllie. 2005. [http://molpharm.aspetjournals.org/cgi/content/full/67/5/1470 Structural features of the glutamate binding site in recombinant NR1/NR2A N-Methyl-D-aspartate receptors determined by site-directed mutagenesis and molecular modeling]. ''Molecular Pharmacology'' 67: 1470-1484. | ||
| + | * Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., ''Prediction of Protein Structures and the Principles of Protein Conformation''. New York: Plenum Press. ISBN 0306431319. | ||
| + | * Dunn, M. S., and B. W. Smart. 1963. [http://www.orgsyn.org/orgsyn/pdfs/CV4P0055.pdf DL-Aspartic Acid]. ''Organic Syntheses'' 4: 55. | ||
| + | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | ||
| + | * Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. ''Lehninger Principles of Biochemistry'', 3rd ed. New York: Worth Publishing. ISBN 1572591536. | ||
| + | |||
| + | |||
==Additional images== | ==Additional images== | ||
Revision as of 18:45, 26 June 2007
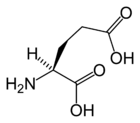 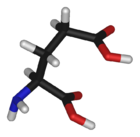
| |
Glutamic acid | |
| Systematic (IUPAC) name | |
| (2S)-2-aminopentanedioic acid | |
| Identifiers | |
| CAS number | 56-86-0 |
| PubChem | 611 |
| Chemical data | |
| Formula | C5H9NO4 |
| Mol. weight | 147.13 |
| SMILES | N[C@@H](CCC(O)=O)C(O)=O |
| Complete data | |
Glutamic acid (Glu, E), also referred to as glutamate (the anion), is one of the 20 proteinogenic amino acids. It is not among the essential amino acids.
Aspartic acid, also called asparaginic acid and alpha-aminosuccinic acid is an acidic, α-amino acid that is found in many proteins, is common in young sugar cane and sugar beets, and is closely related to the amino acid asparagine. Along with glutamic acid, it is classified as an acidic amino acid.
In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids required for normal functioning. However, it is considered to be non-essential since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions.
Aspartic acid is pervasive in biosynthesis and is the precursor to several amino acids. Aspartic acid is a metabolite in the urea cycle and participates in gluconeogenesis. It also acts as a neurotransmitter. The non-carbohydrate, non-nutritive artificial sweetener and flavor enhancer aspartame (aspartyl-phenylalanine-1-methyl ester) is synthesized from aspartic acid and the essential amino acid, phenylalanine.
The discovery, manufacture, and use of the sweetener aspartame, which is now found in many products, represents an aspect of human creativity, addressing a human desire for sweet things while trying to avoid the deleterious health consequences traced to overconsumption of sugar. However, human creativity can be for good or bad, and some health risks have been alleged for aspartame.
Aspartic acid's three letter code is ASP, its one letter code is D, its codons are GAU and GAC, and its systematic name is 2-Aminobutanedioic acid (IUPAC-IUB 1983).
A three-letter designation for either Gln or Glu is Glx—this is often used in cases in which peptide sequencing reactions may convert glutamine to glutamate (or vice versa), leaving the original identity of the amino acid in doubt. The one-letter abbreviation is E for glutamic acid and Q for glutamine.
Glutamic acid Glud E d 2-Aminopentanedioic acid HOOC-[CH2]2-CH(NH2)-COOH
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In aspartic acid, only the L-stereoisomer is involved in protein synthesis.
Aspartic acids chemical formula is HOOC-CH(NH2)-CH2-COOH, or more generally C4H7NO4.
Aspartic acid behaves similarly to glutamic acid. It carries a hydrophilic acidic group with strong negative charge. Aspartic acid usually is located on the outer surface of the protein, making it water-soluble. It binds to positively-charged molecules and ions, often used in enzymes to fix the metal ion.
As its name indicates, it is acidic, with a carboxylic acid component to its side chain. Generally either the amino group will be protonated or one or both of the carboxylic groups will be deprotonated. At neutral pH all three groups are ionized and the species has a charge of -1. The pKa value for Glutamic acid is 4.1. This means that at pH below this value it will be protonated (COOH) and at pH above this value it will be deprotonated (COO-)
Synthesis
Natural
| Reactants | Products | Enzymes |
|---|---|---|
| Glutamine + H2O | → Glu + NH3 | GLS, GLS2 |
| NAcGlu + H2O | → Glu + Acetate | (unknown) |
| α-ketoglutarate + NADPH + NH4+ | → Glu + NADP+ + H2O | GLUD1, GLUD2 |
| α-ketoglutarate + α-amino acid | → Glu + α-oxo acid | transaminase |
| 1-pyrroline-5-carboxylate + NAD+ + H2O | → Glu + NADH | ALDH4A1 |
| N-formimino-L-glutamate + FH4 | ⇌ Glu + 5-formimino-FH4 | FTCD |
Function
In metabolism
Glutamate is a key molecule in cellular metabolism. In humans, dietary proteins are broken down by digestion into amino acids, which serves as metabolic fuel for other functional roles in the body. A key process in amino acid degradation is transamination, in which the amino group of an amino acid is transferred to an α-ketoacid, typically catalysed by a transaminase. The reaction can be generalised as such:
- R1-amino acid + R2-α-ketoacid ⇌ R1-α-ketoacid + R2-amino acid
A very common α-ketoacid is α-ketoglutarate, an intermediate in the citric acid cycle. When α-ketoglutarate undergoes transamination, it always results in glutamate being formed as the corresponding amino acid product. The resulting α-ketoacid product is often a useful one as well, which can contribute as fuel or as a substrate for further metabolism processes. Examples are as follows:
- aspartate + α-ketoglutarate ⇌ oxaloacetate + glutamate
Both pyruvate and oxaloacetate are key components of cellular metabolism, contributing as substrates or intermediates in fundamental processes such as glycolysis, gluconeogenesis and also the citric acid cycle.
Glutamate also plays an important role in the body's disposal of excess or waste nitrogen. Glutamate undergoes deamination, an oxidative reaction catalysed by glutamate dehydrogenase, as follows:
- glutamate + water + NAD+ → α-ketoglutarate + NADH + ammonia + H+
Ammonia (as ammonium) is then excreted predominantly as urea, synthesised in the liver. Transamination can thus be linked to deamination, effectively allowing nitrogen from the amine groups of amino acids to be removed, via glutamate as an intermediate, and finally excreted from the body in the form of urea.
As a neurotransmitter
Glutamate is the most abundant fast excitatory neurotransmitter in the mammalian nervous system. At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger release of glutamate from the pre-synaptic cell. In the opposing post-synaptic cell, glutamate receptors, such as the NMDA receptor, bind glutamate and are activated. Because of its role in synaptic plasticity, it is believed that glutamic acid is involved in cognitive functions like learning and memory in the brain.
Note: As a neurotransmitter, aspartate (the conjugate base of aspartic acid) stimulates NMDA receptors, though not as strongly as the amino acid neurotransmitter glutamate does (Chen et al. 2005). It serves as an excitatory neurotransmitter in the brain and is an excitotoxin.
Glutamate transportersTemplate:Ref N are found in neuronal and glial membranes. They rapidly remove glutamate from the extracellular space. In brain injury or disease, they can work in reverse and excess glutamate can accumulate outside cells. This process causes calcium ions to enter cells via NMDA receptor channels, leading to neuronal damage and eventual cell death, and is called excitotoxicity. The mechanisms of cell death include:
- Damage to mitochondria from excessively high intracellular Ca2+Template:Ref N.
- Glu/Ca2+-mediated promotion of transcription factors for pro-apoptotic genes, or downregulation of transcription factors for anti-apoptotic genes.
Excitotoxicity due to glutamate occurs as part of the ischemic cascade and is associated with stroke and diseases like amyotrophic lateral sclerosis, lathyrism, and Alzheimer's disease.
Glutamic acid has been implicated in epileptic seizures. Microinjection of glutamic acid into neurons produces spontaneous depolarisations around one second apart, and this firing pattern is similar to what is known as paroxysmal depolarizing shift in epileptic attacks. This change in the resting membrane potential at seizure foci could cause spontaneous opening of voltage activated calcium channels, leading to glutamic acid release and further depolarization.
Experimental techniques to detect glutamate in intact cells include using a genetically-engineered nanosensorTemplate:Ref N. The sensor is a fusion of a glutamate-binding protein and two fluorescent proteins. When glutamate binds, the fluorescence of the sensor under ultraviolet light changes by resonance between the two fluorophores. Introduction of the nanosensor into cells enables optical detection of the glutamate concentration. Synthetic analogs of glutamic acid that can be activated by ultraviolet light have also been describedTemplate:Ref N. This method of rapidly uncaging by photostimulation is useful for mapping the connections between neurons, and understanding synapse function.
In brain nonsynaptic glutamatergic signaling circuits
Extracellular glutamate in Drosophila brains has been found to regulate postsynaptic glutamate receptor clustering, via a process involving receptor desensitizationTemplate:Ref N. A gene expressed in glial cells actively transports glutamate into the extracellular spaceTemplate:Ref N, while in the nucleus accumbens stimulating group II metabotropic glutamate receptors was found to reduce extracellular glutamate levelsTemplate:Ref N. This raises the possibility that this extracellular glutamate plays an "endocrine-like" role as part of a larger homeostatic system.
GABA precursor
Glu also serves as the precursor for the synthesis of the inhibitory GABA in GABA-ergic neurons. This reaction is catalyzed by GAD, glutamic acid decarboxylase, which is most abundant in cerebellum and pancreas.
Stiff-man syndrome is a neurologic disorder caused by anti-GAD antibodies, leading to a decrease in GABA synthesis and therefore, impaired motor function such as muscle stiffness and spasm. Since the pancreas is also abundant for the enzyme GAD, a direct immunological destruction occurs in the pancreas and the patients will have diabetes mellitus.
Sources and absorption
Glutamic acid is present in a wide variety of foods and is responsible for one of the five basic tastes of the human sense of taste (umami), especially in its physiological form, the sodium salt of glutamate in a neutral pH. Ninety-five percent of the dietary glutamate is metabolized by intestinal cells in a first pass Template:Ref N.
Overall, glutamic acid is the single largest contributor to intestinal energy. As a source for umami, the sodium salt of glutamic acid, monosodium glutamate (MSG) is used as a food additive to enhance the flavor of foods, although an identical effect can be achieved by mixing and cooking together different ingredients rich in this amino acid and other umami substances as well.
Another source of MSG is fruits, vegetables and nuts that have been sprayed with Auxigro. Auxigro is a growth enhancer that contains 30% glutamic acid.
China-based Fufeng Group Limited is the largest producer of Glutamic Acid in the world, with capacity increasing to 300,000 tons at the end of 2006 from 180,000 tons during 2006, putting them at 25 - 30% of the chinese market. Meihua is the second largest Chinese producer. Together, the top five producers have roughly 50% share in China. Chinese demand is roughly 1.1 million tons per year, while global demand, including China, is 1.7 million tons per year.
Pharmacology
The drug phencyclidine (more commonly known as PCP) antagonizes glutamic acid non-competitively at the NMDA receptor. For the same reasons, sub-anaesthetic doses of Ketamine have strong dissociative and hallucinogenic effects. Glutamate does not easily pass the blood brain barrier, but: "glutamate flux from plasma into brain is mediated by a high affinity transport system at the Blood-Brain Barrier" [1]. It can also be converted into glutamine.
Glutamate transport and supply are obvious targets for the treatment of epilepsy, therefore. In particular Glutamate Restriction Diets are now claiming success anecdotally, by limiting or eliminating intake of wheat, peanut, soy and bean. No similar diets for schizophrenia are known.
ReferencesISBN links support NWE through referral fees
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry, 4th edition.
- Template:Note N File:Free text.png Okumoto, S., et al. (2005). Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proceedings of the National Academy of Sciences U.S.A 102 (24): 8740-8745. PMID 15939876. Template:PMID free
- Template:Note N Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev. 2004 Jul; 45(3):250-65. PubMed
- Template:Note N Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989 Jul;36(1):106-12; PubMed
- Template:Note N File:Free text.png Reeds, P.J., et al. (2000). Intestinal glutamate metabolism. Journal of Nutrition 130 (4s): 978S-982S. PMID 10736365.. Free text
- Template:Note N File:Free text.png Corrie, J.E., et al. (1993). Postsynaptic activation at the squid giant synapse by photolytic release of L-glutamate from a 'caged' L-glutamate. Journal of Physiology 465 (Jun): 1-8. PMID 7901400. Free text
- Template:Note N Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE (2007). Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. Journal of Neuroscience 27 (1): 111-123. PMID 17202478.
- Template:Note N Zheng Xi, Baker DA, Shen H, Carson DS, Kalivas PW (2002). Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. Journal of Pharmacology and Experimental Therapeutics 300 (1): 162-171. PMID 11752112.
- Chen, P. E., M. T. Geballe, P. J. Stansfeld, A. R. Johnston, H. Yuan, A. L. Jacob, J. P. Snyder, S. F. Traynelis, and D. J. A. Wyllie. 2005. Structural features of the glutamate binding site in recombinant NR1/NR2A N-Methyl-D-aspartate receptors determined by site-directed mutagenesis and molecular modeling. Molecular Pharmacology 67: 1470-1484.
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319.
- Dunn, M. S., and B. W. Smart. 1963. DL-Aspartic Acid. Organic Syntheses 4: 55.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
Additional images
- Glutaminsäure - Glutamic acid.svg
Glutamic acid
External links
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.