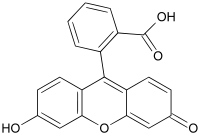
Fluorescein
| Fluorescein | |
|---|---|
| |
| Systematic name | Fluorescein |
| Chemical formula | C20H12O5 |
| Molecular mass | 332.32 g/mol |
| Density | x.xxx g/cm3 |
| Melting point | 314-316°C |
| Boiling point | xx.x °;l/ol |
| CAS number | [2321-07-5] |
| SMILES | OC(C1=C(C(C(C=CC(O)=C3)=C3O2)=C4C2=CC(C=C4)=O)C=CC=C1)=O |
| Disclaimer and references | |
| Fluorescein Isothiocyanate | |
|---|---|
| Fluorescein Isothiocyanate | |
| Systematic name | Fluorescein Isothiocyanate |
| Chemical formula | C21H11NO5S |
| Molecular mass | xx.xx g/mol |
| Density | x.xxx g/cm3 |
| Melting point | >360 °C |
| Boiling point | xx.x °C |
| CAS number | [27072-45-3] |
| SMILES | xxxxx |
| Disclaimer and references | |
Fluorescein is a fluorophore commonly used in microscopy, in a type of dye laser as the gain medium, in forensics and serology to detect latent blood stains, and in dye tracing. Fluorescein has an absorption maximum at 490 nm and emission maximum of 514 nm (in water). Also, fluorescein has an isoabsorptive point (equal absorption for all pH values) at 460 nm.
Chemical and physical properties

The fluorescence of this molecule is very high, and excitation occurs at 494 nm and emission at 525 nm.
Fluorescein has a pKa at 6.4 and multiple ionization equilibria. This leads to pH dependent absorption and emission over the range of 5 to 9. Also, the fluorescence lifetimes of the protonated and deprotonated forms of fluorescein are approximately 3 and 4 ns, which allows for pH determination from non-intensity based measurements. The lifetimes can be recovered using time-correlated single photon counting or phase-modulation fluorimetry.
There are many fluorescein derivatives, for example fluorescein isothiocyanate, often abbreviated as FITC. FITC is the original fluorescein molecule functionalized with an isothiocyanate group (-N=C=S), replacing a hydrogen atom on the bottom ring of the structure. This derivative is reactive towards amine groups on proteins inside cells. Other derivatives include Oregon Green, Tokyo Green, SNAFL, and carboxynaphthofluorescein. These have been tailored for various chemical and biological applications where higher photostability, different spectral characteristics, or different attachment groups are needed.
Synthesis
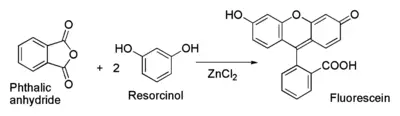
Fluorescein can be prepared from phthalic anhydride and resorcinol in the presence of zinc chloride via the Friedel-Crafts reaction.
A second method to prepare fluorescein uses methanesulfonic acid as the catalyst.
Applications
Uses in river systems
One of its more recognizable uses is in the Chicago River, where fluorescein is used to dye the river green on St. Patrick's Day.
Other uses of fluorescein include using it as a water-soluble dye added to rainwater in environmental testing simulations to aid in locating and analyzing any water leaks, and in Australia and New Zealand as a methylated spirit dye.
Biological research
In biology, the isothiocyanate derivative of fluorescein is often used to label and track cells in fluorescence microscopy applications. Additional biologically active molecules (such as antibodies) may also be attached to fluorescein, allowing biologists to target the fluorophore to specific proteins or structures within cells. This application is common in yeast display.
Fluorescein can also be conjugated to nucleoside triphosphates and incorporated into a probe for in situ hybridisation. Fluorescein-labelled probes can be imaged using FISH, or targeted by antibodies using immunohistochemistry. The latter is a common alternative to digoxigenin, and the two are used together for labelling two genes in one sample.
Ophthalmic applications
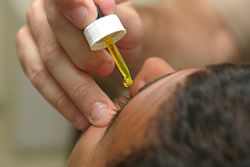
Fluorescein sodium is used extensively as a diagnostic tool in the field of ophthalmology. It is applied topically in the form of a drop or it can be injected intravenously to produce a fluorescein angiogram.
Topical fluorescein is useful in the diagnosis of corneal abrasions, corneal ulcers, herpetic corneal infections, and dry eye. Fluorescein angiography is used to diagnose and categorize macular degeneration, diabetic retinopathy, inflammatory intraocular conditions, and intraocular tumors.
External links
- Absorption and Emission Spectra of Fluorescein in Ethanol
- Absorption and Emission Spectra of Fluorescein in Basic Ethanol
- Fluorescein Ionization Equilibria
Template:ChemicalSources
ca:Fluoresceïna de:Fluorescein fr:fluorescéine pl:Fluoresceina ru:Флуоресцеин sv:Fluorescein