Difference between revisions of "Ethyl acetate" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
|||
| Line 1: | Line 1: | ||
| + | {{Claimed}} | ||
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
! {{chembox header}} | Ethyl acetate | ! {{chembox header}} | Ethyl acetate | ||
| Line 13: | Line 14: | ||
|- | |- | ||
| Other names | | Other names | ||
| − | | ethyl ester,</ | + | | ethyl ester,<br/> ethyl acetate,<br/> acetic ester,<br/> ester of ethanol<br/> |
|- | |- | ||
| [[Chemical formula|Molecular formula]] | | [[Chemical formula|Molecular formula]] | ||
| Line 38: | Line 39: | ||
| 8.3 g/100 mL (20 °C) | | 8.3 g/100 mL (20 °C) | ||
|- | |- | ||
| − | | [[Soluble|Solubility]] in [[ethanol]],</ | + | | [[Soluble|Solubility]] in [[ethanol]],<br/> [[acetone]], [[diethyl ether]],<br/> [[benzene]] |
| Miscible | | Miscible | ||
|- | |- | ||
| Line 87: | Line 88: | ||
|- | |- | ||
| [[Ethyl acetate (data page)#Thermodynamic properties|Thermodynamic<br/>data]] | | [[Ethyl acetate (data page)#Thermodynamic properties|Thermodynamic<br/>data]] | ||
| − | | Phase behaviour<br>Solid, liquid, gas | + | | Phase behaviour<br/>Solid, liquid, gas |
|- | |- | ||
| [[Ethyl acetate (data page)#Spectral data|Spectral data]] | | [[Ethyl acetate (data page)#Spectral data|Spectral data]] | ||
| Line 95: | Line 96: | ||
|- | |- | ||
| Related [[carboxylate ester]]s | | Related [[carboxylate ester]]s | ||
| − | | [[Methyl acetate]],<br>[[Butyl acetate]] | + | | [[Methyl acetate]],<br/>[[Butyl acetate]] |
|- | |- | ||
| Related compounds | | Related compounds | ||
| [[Acetic acid]],<br/>[[ethanol]] | | [[Acetic acid]],<br/>[[ethanol]] | ||
|- | |- | ||
| − | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br> materials in their [[standard state|standard state (at 25 °C, 100 kPa)]]<br/>[[wikipedia:Chemical infobox|Infobox disclaimer and references]]</small> | + | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br/> materials in their [[standard state|standard state (at 25 °C, 100 kPa)]]<br/>[[wikipedia:Chemical infobox|Infobox disclaimer and references]]</small> |
|- | |- | ||
|} | |} | ||
| Line 107: | Line 108: | ||
==Properties== | ==Properties== | ||
| − | Ethyl acetate is a moderately polar solvent that has the advantages of being [[Volatility (chemistry)|volatile]], relatively non-toxic, and non-[[hygroscopic]]. It is a weak [[hydrogen bond]] acceptor, and is not a donor due to the lack of an [[acidic]] proton (one directly bonded to an [[electronegative]] atom such as fluorine, oxygen, or nitrogen). Ethyl acetate can dissolve up to 3% [[water]] and has a [[solubility]] of 8% in water at room temperature. At elevated temperature its | + | Ethyl acetate is a moderately polar solvent that has the advantages of being [[Volatility (chemistry)|volatile]], relatively non-toxic, and non-[[hygroscopic]]. It is a weak [[hydrogen bond]] acceptor, and is not a donor due to the lack of an [[acidic]] proton (one directly bonded to an [[electronegative]] atom such as fluorine, oxygen, or nitrogen). Ethyl acetate can dissolve up to 3% [[water]] and has a [[solubility]] of 8% in water at room temperature. At elevated temperature its solubility in water is higher. It is unstable in the presence of strong aqueous [[Base (chemistry)|bases]] and [[acid]]s. |
==Uses and occurrence== | ==Uses and occurrence== | ||
| Line 118: | Line 119: | ||
===Other uses=== | ===Other uses=== | ||
| + | |||
In the field of [[entomology]], ethyl acetate is an effective poison for use in [[insect collecting]] and study. In a [[killing jar]] charged with ethyl acetate, the vapors will kill the collected (usually adult) insect quickly without destroying it. Because it is not [[hygroscopic]], ethyl acetate also keeps the insect soft enough to allow proper mounting suitable for a collection. | In the field of [[entomology]], ethyl acetate is an effective poison for use in [[insect collecting]] and study. In a [[killing jar]] charged with ethyl acetate, the vapors will kill the collected (usually adult) insect quickly without destroying it. Because it is not [[hygroscopic]], ethyl acetate also keeps the insect soft enough to allow proper mounting suitable for a collection. | ||
==Synthesis== | ==Synthesis== | ||
| + | |||
Ethyl acetate is synthesized via the [[Fischer esterification]] reaction from [[acetic acid]] and [[ethanol]], typically in the presence of an acid catalyst such as [[sulfuric acid]]. | Ethyl acetate is synthesized via the [[Fischer esterification]] reaction from [[acetic acid]] and [[ethanol]], typically in the presence of an acid catalyst such as [[sulfuric acid]]. | ||
:CH<sub>3</sub>CH<sub>2</sub>OH + CH<sub>3</sub>COOH → CH<sub>3</sub>COOCH<sub>2</sub>CH<sub>3</sub> + [[Water (molecule)|H<sub>2</sub>O]] | :CH<sub>3</sub>CH<sub>2</sub>OH + CH<sub>3</sub>COOH → CH<sub>3</sub>COOCH<sub>2</sub>CH<sub>3</sub> + [[Water (molecule)|H<sub>2</sub>O]] | ||
| Line 133: | Line 136: | ||
==See also== | ==See also== | ||
| + | |||
| + | * [[Acetic acid]] | ||
| + | * [[Ethanol]] | ||
* [[Solvent]] | * [[Solvent]] | ||
| + | |||
| + | == References == | ||
| + | |||
| + | * McMurry, John. 2004. ''Organic Chemistry''. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052. | ||
| + | |||
| + | * Morrison, Robert T., and Robert N. Boyd. 1992. ''Organic Chemistry''. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2. | ||
| + | |||
| + | * Solomons, T.W. Graham, and Fryhle, Craig B. 2004. ''Organic Chemistry''. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998. | ||
==External links== | ==External links== | ||
| + | |||
* [http://www.jtbaker.com/msds/englishhtml/e2850.htm Material safety data (MSDS)] for ethyl acetate | * [http://www.jtbaker.com/msds/englishhtml/e2850.htm Material safety data (MSDS)] for ethyl acetate | ||
* [http://www.npi.gov.au/database/substance-info/profiles/38.html National Pollutant Inventory - Ethyl acetate fact sheet] | * [http://www.npi.gov.au/database/substance-info/profiles/38.html National Pollutant Inventory - Ethyl acetate fact sheet] | ||
| Line 141: | Line 156: | ||
* [http://hsc.csu.edu.au/chemistry/core/acidic/chem935/chem935net.html#net4 Purpose of Using Concentrated Sulfuric Acid in Esterification for Catalysis] | * [http://hsc.csu.edu.au/chemistry/core/acidic/chem935/chem935net.html#net4 Purpose of Using Concentrated Sulfuric Acid in Esterification for Catalysis] | ||
| + | [[Category:Physical sciences]] | ||
| + | [[Category:Chemistry]] | ||
| + | [[Category:Organic chemistry]] | ||
| − | + | {{credit|156526580}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 18:17, 22 September 2007
| Ethyl acetate | |
|---|---|
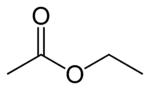 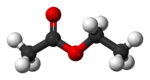
| |
| General | |
| IUPAC name | Ethyl acetate |
| Systematic name | Ethyl ethanoate |
| Other names | ethyl ester, ethyl acetate, acetic ester, ester of ethanol |
| Molecular formula | C4H8O2 |
| SMILES | CCOC(C)=O |
| Molar mass | 88.105 g/mol |
| Appearance | colorless liquid |
| CAS number | [141-78-6] |
| Properties | |
| Density and phase | 0.897 g/cm³, liquid |
| Solubility in water | 8.3 g/100 mL (20 °C) |
| Solubility in ethanol, acetone, diethyl ether, benzene |
Miscible |
| Melting point | −83.6 °C (189.55 K) |
| Boiling point | 77.1 °C (350.25 K) |
| Critical temperature | 250.11 °C (523.26 K) |
| Viscosity | 0.426 cP at 25 °C |
| Structure | |
| Dipole moment | 1.78 D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Flammable (F), Irritant (Xi) |
| NFPA 704 | |
| R-phrases | R11, R36, R66, R67 |
| S-phrases | S16, S26, S33 |
| Flash point | −4 °C |
| RTECS number | AH5425000 |
| Supplementary data page | |
| Structure and properties |
n = 1.3720 |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related carboxylate esters | Methyl acetate, Butyl acetate |
| Related compounds | Acetic acid, ethanol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Ethyl acetate is the organic compound with the formula CH3CH2OC(O)CH3. This colorless liquid has a characteristic, not unpleasant smell (similar to pear drops) like certain glues or nail polish removers , in which it is used. As the ester derived from ethanol and acetic acid, EtOAc, as it is commonly abbreviated, is manufactured on a large scale for use as a solvent.
Properties
Ethyl acetate is a moderately polar solvent that has the advantages of being volatile, relatively non-toxic, and non-hygroscopic. It is a weak hydrogen bond acceptor, and is not a donor due to the lack of an acidic proton (one directly bonded to an electronegative atom such as fluorine, oxygen, or nitrogen). Ethyl acetate can dissolve up to 3% water and has a solubility of 8% in water at room temperature. At elevated temperature its solubility in water is higher. It is unstable in the presence of strong aqueous bases and acids.
Uses and occurrence
Ethyl acetate is widely employed as a solvent for nail varnishes and nail varnish removers. Industrially it is used to decaffeinate coffee beans and tea leaves.[citation needed] In chemistry, it is often mixed with a non-polar solvent such as hexanes as a chromatography solvent. It is also used as a solvent for extractions. It is rarely used as a reaction mixture due to the weakness of the ester linkage — it hydrolyzes in the presence of strong acids and bases to give acetic acid and ethanol.
Ethyl acetate is also present in confectionery, perfumes, and fruits. It is used in perfumes because it evaporates at a fast rate, leaving but the scent of the perfume on the skin. It also confers a fruity smell, as do most esters. It is also used in paints as an activator or hardener.
Occurrence in wines
Ethyl acetate is present in wines. It may be considered a contaminant at too high concentrations, as typically occurs when wine is exposed to air for a prolonged period. When present at too high concentration in wine, it is regarded as an off-flavour.
Other uses
In the field of entomology, ethyl acetate is an effective poison for use in insect collecting and study. In a killing jar charged with ethyl acetate, the vapors will kill the collected (usually adult) insect quickly without destroying it. Because it is not hygroscopic, ethyl acetate also keeps the insect soft enough to allow proper mounting suitable for a collection.
Synthesis
Ethyl acetate is synthesized via the Fischer esterification reaction from acetic acid and ethanol, typically in the presence of an acid catalyst such as sulfuric acid.
- CH3CH2OH + CH3COOH → CH3COOCH2CH3 + H2O
Because the reaction is reversible and produces an equilibrium, the yield is low unless water is removed. In the laboratory, the ethyl acetate product can be isolated from water using a Dean-Stark apparatus.
Reactions
Ethyl acetate can be hydrolyzed in acid or basic conditions to regain acetic acid and ethanol. The use of an acid catalyst such as sulfuric acid gives poor yields due to it being an equilibrium — the reverse reaction of the Fischer esterification.
To obtain high yields, it is preferable to use a stoichiometric amount of strong base, such as sodium hydroxide. This reaction gives ethanol and sodium acetate, which is not able to react with ethanol any longer:
- CH3CO2C2H5 + NaOH → C2H5OH + CH3CO2Na
See also
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2.
- Solomons, T.W. Graham, and Fryhle, Craig B. 2004. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
External links
- Material safety data (MSDS) for ethyl acetate
- National Pollutant Inventory - Ethyl acetate fact sheet
- Ethyl Acetate: Molecule of the Month
- Purpose of Using Concentrated Sulfuric Acid in Esterification for Catalysis
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
