Tyrosine
| Tyrosine | |
|---|---|
| |
| Systematic name | (S)-2-Amino-3-(4-hydroxy- phenyl)-propanoic acid |
| Abbreviations | Tyr Y |
| Chemical formula | C9H11NO3 |
| Molecular mass | 181.19 g mol-1 |
| Melting point | 343 °C |
| Density | 1.456 g cm-3 |
| Isoelectric point | 5.66 |
| pKa | 2.24 9.04 10.10 |
| Molar extinction coefficient | 1420 M-1 cm-1 at 274.6 nm |
| PubChem | 1153 |
| CAS number | [60-18-4] |
| EINECS number | 200-460-4 |
| SMILES | N[C@@H](Cc1ccc(O)cc1)C(O)=O |
Absorption and emission spectrum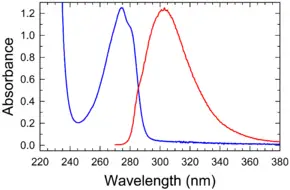
Absorbance and fluorescence of tyrosine in water/buffer | |
| Disclaimer and references | |
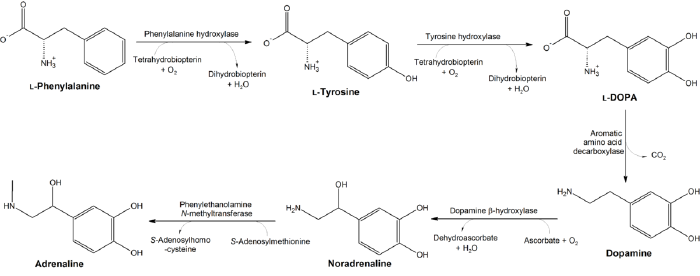
Tyrosine is an α-amino acid that is found in most proteins (such as insulin), is normally readily converted from the essential amino acid phenylalanine in the human body, and is a precursor of such important chemical compounds as epinephrine (adrenaline), norepinephrine (noradrenaline), dopamine, thyroid hormones, and melanin.
In humans, the L-isomer of tyrosine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. However, tyrosine is considered to be a "non-essential amino acid" since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions—in this case, synthesized from phenylalanine. Tyrosine, phenylalanine, and tryptophan are the biggest of the standard amino acids.
The human body involves intricate coordination of parts and processes, as exemplified by tyrosine production from phenylalanine and by the metabolism of tyrosine to produce other important products. Catalyzed by enzymes, l-phenylalanine is degraded into l-tyrosine, which in turn is converted into L-DOPA, which is further metabolized into dopamine, norepinephrine, and epinephrine. However, in the advent of the lack of particular enzymes due to a genetic defect, this delicate harmony and balance is disrupted. In the case of the genetic disorder phenylketonuria, the body loses its ability to metabolize phenylalanine. In the case of alkaptonuria, there is a disorder of tyrosine metabolism.
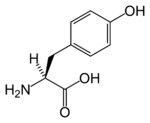
Tyrosine's three letter code is Tyr, its one letter code is Y, and its systematic name is 2-Amino-3-(4-hydroxyphenyl)-propanoic acid (IUPAC-IUB 1983). It is also known as 4-hydroxyphenylalanine.
The name tyrosine is derived from the Greek tyros, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in cheese, obtained as a degradation product of the protein casein.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In tyrosine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
Tyrosine's chemical formula is C9H11NO3 (IUPAC-IUB 1983) (that is, one more nitrogen atom than phenylalanine).
Like phenylalanine and tryptophan, tyrosine contains a large rigid aromatic group on the side chain; in the case of tyrosine, a phenol side chain with a hydroxyl group. Tyrosine, phenylalanine, and tryptophan—like isoleucine, leucine, and valine—are hydrophobic and tend to orient towards the interior of the folded protein molecule.
Isomers
Based on the location of the hydroxyl group on the side chain, there are three structural isomers of tyrosine, namely para-tyrosine (p-Tyr), meta-tyrosine (m-Tyr), and ortho-tyrosine (o-Tyr). Enzymatically, only the first isomer (p-Tyr) is produced from L-phenylalanine by the phenylalanine-hydroxylase enzyme. The other two isoforms, m-Tyr and o-Tyr, can be produced as a consequence of free radical attack on phenylalanine in states with increased oxidative stress.
Biosynthesis
Tyrosine cannot be completely synthesized by animals, although it can be made by hydroxylation of phenylalanine if the latter is in abundant supply.
It is synthesized by plants and most microorganisms from prephenate, an intermediate in the biosynthesis of both tyrosine and phenylalanine on the shikimate pathway.
Prephenate is oxidatively decarboxylated with retention of the hydroxyl group to give p-hydroxyphenylpyruvate. This is transaminated using glutamate as the nitrogen source to give tyrosine and α-ketoglutarate.
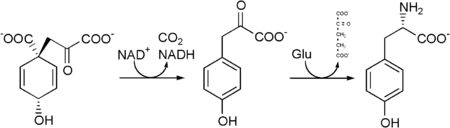
In the process used by animals to convert phenylalanine to tryosine, the enzyme phenylalanine hydroxylase is utilized. If this reaction does not take place due to a genetic lack of this enzyme, then phenylalanine accumulates and tyrosine is deficient. This serious disorder is known as phenylketonuria.
Biological aspects
As noted, L-phenylalanine can be converted into L-tyrosine, utilizing the enzyme phenylalanine hydroxylase. In turn, L-tyrosine is converted to levodopa (L-DOPA) by the enzyme tyrosine hydroxylase. This can be further converted into dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) (the latter three are known as catecholamines).
Tyrosine hydroxylase (TH) is the rate-limiting enzyme involved in the synthesis of the catecholamines dopamine, norepinephrine, and epinephrine.
Tyrosine is also precursor to the thyroid hormones thyroxine and triiodothyronine and the pigment melanin.
Medical use
L-tyrosine is sometimes recommended by practitioners as helpful for weight loss, clinical depression, Parkinson's Disease, and phenylketonuria; however, one study found that it had no impact on endurance exercise performance (Chinevere et al. 2002).
Alkaptonuria
Alkaptonuria (black urine disease, alcaptonuria, or ochronosis) is a rare inherited genetic disorder of tyrosine metabolism. This is an autosomal recessive trait that is caused by a defect in the enzyme homogentisic acid oxidase (EC 1.13.11.5). The enzyme normally breaks down a toxic tyrosine byproduct, homogentisic acid (also called alkapton), which is harmful to bones and cartilage and is excreted in urine.
A distinctive characteristic of alkaptonuria is that ear wax exposed to air turns red or black (depending on diet) after several hours because of the accumulation of homogentisic acid. Similarly, urine exposed to air can become dark; this is useful for diagnosing young children using diapers. In adulthood, but usually not before age forty, persons suffering from alkaptonuria develop progressive arthritis (especially of the spine), due to the long-term buildup of homogentisate in bones and cartilage. The urine is malodorous.
Prevention is not possible and the treatment is aimed at ameliorating symptoms. Reducing intake of the amino acids phenylalanine and tyrosine to the minimum required to sustain health (phenylalanine is an essential amino acid) can help slow the progression of the disease.
Phenylketonuria
Phenylketonuria (PKU) is an autosomal recessive genetic disorder characterized by a deficiency in the enzyme phenylalanine hydroxylase (PAH). This enzyme is necessary to metabolize the amino acid phenylalanine to tyrosine. When PAH is deficient, phenylalanine accumulates and is converted into phenylketones, which are detected in the urine. These include phenylacetate, phenylpyruvate, and phenylethylamine (Michals and Matalon 1985). Detection of phenylketones in the urine is diagnostic.
Phenylalanine is a large, neutral amino acid (LNAA). LNAAs compete for transport across the blood brain barrier (BBB) via the large neutral amino acid transporter (LNAAT). Excessive phenylalanine in the blood saturates the transporter. Thus, excessive levels of phenylalanine significantly decrease the levels of other LNAAs in the brain. But since these amino acids are required for protein and neurotransmitter synthesis, phenylalanine accumulation disrupts brain development in children, leading to mental retardation (Pietz et al. 1999)
Individuals with this disorder are known as "phenylketonurics." Left untreated, this condition can cause problems with brain development, leading to progressive mental retardation and seizures. However, PKU is one of the few genetic diseases that can be controlled by diet. A diet low in phenylalanine and high in tyrosine can bring about a nearly total cure.
ReferencesISBN links support NWE through referral fees
- Chinevere, T. D., R. D. Sawyer, A. R. Creer, R. K. Conlee, and A. C. Parcell. 2002. Effects of L-tyrosine and carbohydrate ingestion on endurance exercise performance. Journal of Applied Physiology 93(5): 1590-1597. Retrieved June 20, 2007.
- Folling, A. 1934. Ueber ausscheidung von phenylbrenztraubensaeure in den harn als stoffwechselanomalie in verbindung mit imbezillitaet. Ztschr. Physiol. Chem. 227: 169-176.
- Hoffhines, A. J., E. Damoc, K. G. Bridges, J. A. Leary, and K. L. Moore. 2006. Detection and purification of tyrosine-sulfated proteins using a novel anti-sulfotyrosine monoclonal antibody. Journal of Biological Chemistry 281: 37877-37887. Retrieved June 20, 2007.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
- Michals, K., and R. Matalon. 1985. Phenylalanine metabolites, attention span and hyperactivity. American Journal of Clinical Nutrition. 42(2): 361-365. PMID 4025205.
- Molnar, G. A., Z. Wagner, L. Markó, T. Kó Szegi, M. Mohás, B. Kocsis, Z. Matus, L. Wagner, M. Tmaskó, I. Mazák, B. Laczy, J. Nagy, and I. Wittmann. 2005. Urinary ortho-tyrosine excretion in diabetes mellitus and renal failure: evidence for hydroxyl radical production. Kidney International 68: 2281-2287. Retrieved June 20, 2007.
- Molnar, G. A., V. Nemes, Z. Biró, A. Ludány, Z. Wagner, and I. Wittmann. 2005. Accumulation of the hydroxyl free radical markers meta-, ortho-tyrosine and DOPA in cataractous lenses is accompanied by a lower protein and phenylalanine content of the water-soluble phase. Free Radical Research 39(12): 1359-1366. Retrieved June 20, 2007.
- Pietz, J., R. Kreis, A. Rupp, E. Mayatepek, D. Rating, C. Boesch, and H. J. Bremer. 1999. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. Journal of Clinical Investigation 103: 1169–1178. PMID 10207169.
External links
All links retrieved May 2, 2023.
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.