Sulfate
In inorganic chemistry, a sulfate (IUPAC-recommended spelling; also sulphate in British English) is a salt of sulfuric acid. The sulfate ion is a polyatomic anion with the empirical formula SO42−.
Sulfate salts have diverse applications. For example, magnesium sulfate (or Epsom salts) is used in therapeutic baths; gypsum, the mineral form of hydrated calcium sulfate, is used to produce plaster; and copper sulfate is an algaecide. Some microorganisms that live near deep-sea thermal vents utilize sulfates as electron acceptors.
Chemical properties
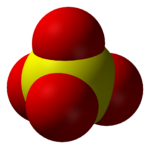
The sulfate ion (SO42−) has a molecular mass of 96.06 daltons. Each anion consists of one central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement.
The sulfate ion is the conjugate base of the hydrogen sulfate (also known as bisulfate) ion, HSO4−. The hydrogen sulfate ion in turn is the conjugate base of sulfuric acid, H2SO4.
Sulfate compounds arise when cations combine with the anion SO42−. Often this combination results in an ionic compound, although sulfates can engage in covalent bonding with most elements. The metal complex PtSO4P(C6H5)32 is clearly covalent Pt-O bonding. Dialkylsulfates, such as dimethylsulfate are covalent, distillable species. Many sulfate salts are highly soluble in water. Exceptions include calcium sulfate, strontium sulfate, and barium sulfate, which are poorly soluble. The barium derivative is useful in the gravimetric analysis of sulfate: one adds a solution of, perhaps, barium chloride to a solution containing sulfate ions. The appearance of a white precipitate, which is barium sulfate, indicates that sulfate anions are present.
Uses
Sulfates are important in both the chemical industry and biological systems. Some uses are listed below.
- Some anaerobic microorganisms, such as those living near deep sea thermal vents, utilize sulfates as electron acceptors.
- Magnesium sulfate, commonly known as Epsom salts, is used in therapeutic baths.
- Gypsum, the natural mineral form of hydrated calcium sulfate, is used to produce plaster.
- Copper sulfate is a common algaecide.
- The sulfate ion is used as counter ion for some cationic drugs.
Environmental effects
Sulfates occur as microscopic particles (aerosols) resulting from fossil fuel and biomass combustion. They increase the acidity of the atmosphere and form acid rain.
Main effects on climate
The first (direct) effect is to scatter light, effectively increasing the Earth's albedo. This effect is moderately well understood and leads to a cooling from the negative radiative forcing of about 0.5 W/m2 relative to pre-industrial values,[1] partially offsetting the larger (about 2.4 W/m2) warming effect of greenhouse gases. The effect is strongly spatially non-uniform, being largest downstream of large industrial areas.
The first indirect effect is also known as the Twomey effect. Sulfate aerosols can act as cloud condensation nuclei and this leads to greater numbers of smaller droplets of water. Lots of smaller droplets can diffuse light more efficiently than just a few larger droplets.
The second indirect effect is the further knock-on effects of having more cloud condensation nuclei. It is proposed that these include the suppression of drizzle, increased cloud height (Pincus & Baker 1994), to facilitate cloud formation at low humidities and longer cloud lifetime (Albrecht 1989). Sulfate may also result in changes in the particle size distribution, which can affect the clouds radiative properties in ways that are not fully understood. Chemical effects such as the dissolution of soluble gases and slightly soluble substances, surface tension depression by organic substances and accommodation coefficient changes are also included in the second indirect effect[2].
The indirect effects probably have a cooling effect, perhaps up to 2 W/m2, although the uncertainty is very large.
Sulfates are therefore implicated in global dimming, which may have acted to offset some of the effects of global warming.
Oxoanions of sulfur
- SO52− persulfate ion
- SO42− sulfate ion
- SO32− sulfite ion
- SO22− hyposulfite ion
- S2O82− peroxydisulfate ion
See Also
- Salt
- Sulfonate
- Sulfuric acid
Notes
- ↑ Figure 3: The global mean radiative forcing of the climate system for the year 2000, relative to 1750 Climate Change 2001: Working Group I: The Scientific Basis. IPCC.
- ↑ Chemical Amplification (or dampening) of the Twomey Effect: Conditions derived from droplet activation theory. T.A. Rissman, A. Nenes, J.H. Seinfeld.
ReferencesISBN links support NWE through referral fees
- Chang, Raymond. Chemistry. 9th ed. New York: McGraw-Hill Science/Engineering/Math, 2006. ISBN 0073221031
- Cotton, F. Albert, and Geoffrey Wilkinson. Advanced Inorganic Chemistry. 4th ed. New York: Wiley, 1980. ISBN 0471027758
- McMurry, J., and R.C. Fay. Chemistry. 4th ed. Upper Saddle River, NJ: Prentice Hall, 2004. ISBN 0131402080
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.