Difference between revisions of "Silicone" - New World Encyclopedia
Rosie Tanabe (talk | contribs) m |
|||
| (11 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Images OK}}{{Submitted}}{{Approved}}{{Paid}}{{Copyedited}} |
{{Distinguish2|the element [[silicon]]}} | {{Distinguish2|the element [[silicon]]}} | ||
[[Image:Caulking.jpg|thumb|200px|Silicone caulk can be used as a basic sealant against water and air penetration.]] | [[Image:Caulking.jpg|thumb|200px|Silicone caulk can be used as a basic sealant against water and air penetration.]] | ||
| − | [[Image: | + | [[Image:Sl_silicone_pipes.jpg|thumb|200px|Self-leveling silicone [[firestop]] [[system]] used around [[copper]] [[pipe (material)|pipe]]s penetrating a two-hour [[Fire-resistance rating|fire-resistance rated]] [[concrete]] floor assembly.]] |
| − | [[ | ||
| − | '''Silicones''' (more accurately called [[polymer]]ized [[siloxane]]s or '''polysiloxanes''') are mixed [[Inorganic compound|inorganic]]-[[Organic compound|organic]] | + | '''Silicones''' (more accurately called [[polymer]]ized [[siloxane]]s or '''polysiloxanes''') are mixed [[Inorganic compound|inorganic]]-[[Organic compound|organic]] [[polymer]]s. Their general [[chemical formula]] can be written as [R<sub>2</sub>SiO]<sub>n</sub>, where [[functional group|R]] corresponds to an organic group such as [[methyl]], [[ethyl]], or [[phenyl]]. By varying their composition and molecular structures, silicones with a range of properties can be prepared. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common siloxane is [[polydimethylsiloxane]] (PDMS), a [[silicone oil]]. The second largest group of silicone materials is based on [[silicone resin]]s. |
| + | {{toc}} | ||
| + | Different types of silicones have been developed for a variety of applications. For instance, they are used as sealants, molds, lubricants, dry cleaning [[solvent]]s, electrical insulators, and protective material for electronic components. They are also found in some firestops, personal care products, and hearing aids. However, their uses in [[breast implant]]s and [[nuclear reactor]] buildings have stirred controversy. | ||
| − | ==Chemical terminology== | + | ==Chemical structure and terminology == |
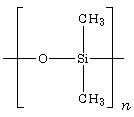
| − | + | [[Image:PDMS.png|thumb|150px|Chemical structure of polydimethylsiloxane (PDMS).]] | |
| − | The word "silicone" is derived from ''[[ketone]]''. | + | Silicone is often mistakenly referred to as "silicon." Although silicones contain [[silicon]] atoms, they are not made up exclusively of silicon, and they have completely different physical characteristics from elemental silicon. |
| + | |||
| + | The word "silicone" is derived from ''[[ketone]]''. Dimethylsilicone and dimethyl ketone ([[acetone]]) have analogous [[chemical formula]]s, therefore it was surmised (incorrectly) that they have analogous structures.<ref>The same terminology is used for compounds such as [[silane]] (an analog of [[methane]]).</ref> In the case of an acetone (or any ketone) molecule, there is a double bond between a [[carbon]] atom and an [[oxygen]] atom. On the other hand, a silicone molecule does not contain a double bond between a silicon atom and an oxygen atom. Chemists have found that the silicon atom forms a single bond with each of two oxygen atoms, rather than a double bond to a single atom. | ||
| + | |||
| + | Polysiloxanes are called "silicones" due to early mistaken assumptions about their structure. They consist of an inorganic [[silicon]]-[[oxygen]] backbone (...-Si-O-Si-O-Si-O-...) with organic side groups attached to the silicon atoms (see the figure showing the structure of polydimethylsiloxane). In some cases, organic side groups can be used to link two or more of these -Si-O- backbones together. | ||
| + | |||
| + | By varying the -Si-O- chain lengths, side groups, and [[Cross-link|crosslinking]], a variety of silicones can be synthesized. The most common siloxane is linear [[polydimethylsiloxane]] (PDMS), a [[silicone oil]] (see the structure shown in the figure). The second largest group of silicone materials is based on [[silicone resin]]s, which are formed by branched and cage-like oligosiloxanes. | ||
==Synthesis== | ==Synthesis== | ||
| − | Silicones are synthesized from [[chlorosilane]]s, [[tetraethoxysilane]], and related compounds. | + | |
| + | Silicones are synthesized from [[chlorosilane]]s, [[tetraethoxysilane]], and related compounds. In the case of PDMS, the starting material is dimethylchlorosilane, which reacts with [[water]] as follows: | ||
:n [Si(CH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>] + n [H<sub>2</sub>O] → [Si(CH<sub>3</sub>)<sub>2</sub>O]<sub>n</sub> + 2n HCl | :n [Si(CH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>] + n [H<sub>2</sub>O] → [Si(CH<sub>3</sub>)<sub>2</sub>O]<sub>n</sub> + 2n HCl | ||
| − | During polymerization, this reaction evolves potentially hazardous [[hydrogen chloride]] gas. | + | During polymerization, this reaction evolves potentially hazardous [[hydrogen chloride]] gas. For medical uses, a process was developed where the chlorine atoms in the silane precursor were replaced with acetate groups, so that the reaction product of the final curing process is nontoxic [[acetic acid]] (vinegar). As a side effect, the curing process is also much slower in this case. This is the chemistry used in many consumer applications, such as silicone [[caulk]] and [[adhesive]]s. |
| − | Silane precursors with more acid-forming groups and fewer methyl groups, such as methyltrichlorosilane, can be used to introduce [[Branching (chemistry)|branches]] or [[cross-link]]s in the polymer chain. | + | Silane precursors with more acid-forming groups and fewer methyl groups, such as methyltrichlorosilane, can be used to introduce [[Branching (chemistry)|branches]] or [[cross-link]]s in the polymer chain. Ideally, each molecule of such a compound becomes a branch point. This can be used to produce hard [[silicone resin]]s. Similarly, precursors with three [[methyl]] groups can be used to limit molecular weight, since each such molecule has only one reactive site and so forms the end of a siloxane chain. |
| − | Modern silicone resins are made with [[tetraethoxysilane]], which reacts in a more | + | Modern silicone resins are made with [[tetraethoxysilane]], which reacts in a milder and more controllable manner than chlorosilanes. |
==Properties== | ==Properties== | ||
| Line 30: | Line 38: | ||
# Excellent resistance to oxygen, ozone, and sunlight | # Excellent resistance to oxygen, ozone, and sunlight | ||
# Flexibility | # Flexibility | ||
| − | # | + | # Electrically insulating or conductive, depending on structure and composition |
# Anti-adhesive | # Anti-adhesive | ||
# Low chemical reactivity | # Low chemical reactivity | ||
# Low toxicity | # Low toxicity | ||
| − | # High gas permeability | + | # High gas permeability<ref>At room temperature (25°C), the permeability of silicone rubber for gases like oxygen is approximately 400 times that of [[butyl]] rubber, making silicone useful for medical applications. At the same time, this property precludes it from applications where gas-tight seals are necessary.</ref> |
| − | == | + | === Silicone rubber === |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | A flexible, rubbery polysiloxane is known as ''silicone rubber''. It can be extruded into tubes, strips, solid cord, and custom profiles. It offers excellent resistance to extreme temperatures and is highly inert toward most chemicals. [[Rubber|Organic rubber]], with a [[carbon]]-to-carbon backbone, is generally susceptible to [[ozone]], [[UV]], heat, and other aging factors. Silicone rubber, by contrast, can withstand the effects of these agents, making it the material of choice in many extreme environments. Given its inertness, it is used in many medical applications, including medical implants. | |
| − | |||
| − | |||
| − | + | Many specialist grades of silicone rubber have these properties: electrical [[conductivity]], low smoke emission, flame retardation, glow in the dark, and resistance to [[steam]], gases, oils, acids, and other chemicals. | |
| − | |||
| − | + | == Uses of Silicone== | |
| + | ===Moldmaking material=== | ||
| − | + | Two-part silicone systems are used to create rubber molds, which can be used for production casting of resins, foams, rubber, and low-temp alloys. A silicone mold generally requires little or no mold release or surface preparation, as most materials do not adhere to the silicone. | |
| − | |||
| − | + | === Sealants === | |
| − | + | One-part silicone sealants are in common use to seal gaps, joints, and crevices in buildings. These silicones cure by absorbing atmospheric moisture. The strength and reliability of silicone rubber is widely acknowledged in the construction industry. | |
| − | + | An excellent use of silicone rubber is for automotive sunroof seals, which have to endure harsh temperatures and other environmental conditions such as ozone, UV light, and pollution, not to mention common automotive cleaners, waxes, and so forth. | |
| − | + | === Lubricant === | |
| − | [[ | + | In the plumbing and automotive fields, silicone [[grease (lubricant)|grease]] is often used as a lubricant. In plumbing, the grease is typically applied to O-rings in faucets and valves. In the automotive field, silicone grease is typically used as a lubricant for [[brake]] components, as it is stable at high temperatures, is insoluble in water, and far less likely than other lubricants to foul brake pads. |
| − | === | + | ===Cooking applications=== |
| − | + | Silicone is also impregnated into [[parchment]] paper and used as a non-stick material for applications such as baking and steaming. The silicone also makes the paper heat- and grease-resistant. This allows the paper to line cookie sheets and act as a replacement for greasing, thereby speeding mass production of baked goods. It is also commonly used in pouch cooking, where ingredients are sealed into a container made of parchment paper and allowed to steam. | |
| − | + | Silicone rubber is used to make utensils (notably [[spatula]]s) and bakeware. | |
| − | Silicone | + | [[Silicone resin]]s are used in heat-resistant dishware. These often resemble [[ceramic]] items but are much less brittle, making them popular for use with babies. |
| − | === | + | ===Electrical and electronic components=== |
| − | + | Automotive spark plug wires are often insulated by multiple layers of silicone. In addition, electronic components are sometimes protected from environmental influences by [[resin casting|enclosing]] them in silicone. This increases their stability against mechanical shock, radiation, and vibration. Silicones are selected over [[polyurethane]] or [[epoxy]] encapsulation when a wide operating temperature range is required (−150 to 600°F). Silicones also have the advantage of little heat increase in the curing process, low toxicity, good electrical properties, and high purity. Therefore they are used when durability and high performance are required of components under demanding conditions, such as for satellites in space. | |
===Silicone breast implants=== | ===Silicone breast implants=== | ||
| − | + | In the 1980s and 1990s, controversy developed around claims that the silicone gel in [[Breast implant#Silicone gel implants|breast implants]] was responsible for a number of systemic health problems, including [[autoimmune diseases]] and cancer. Multiple lawsuits claiming injury from implants resulted in the 1998 [[bankruptcy]] of [[Dow Corning]] and a moratorium on the use of silicone implants for breast augmentation in the US and Canada pending study. However, multiple studies and expert review panels performed worldwide since then have consistently concluded that women with silicone breast implants are no more likely to develop systemic illness than women without breast implants. In 2006, both [[Health Canada]] and the U.S. [[Food and Drug Administration]] (FDA) adopted positions similar to other countries in permitting the use of silicone implants for cosmetic breast augmentation in their respective countries. | |
===Firestops=== | ===Firestops=== | ||
| + | [[Image:Silicone_foam_sakno1.jpg|thumb|200px|Faulty Sakno silicone foam [[firestop]] installation in the [[Calgary]] sewage treatment plant in the 1980s, used to seal the opening above a [[fire door]] in a cast [[concrete]] [[fire]] separation.]] | ||
| − | + | When properly installed, silicone foam [[firestop]]s can be fabricated for building code compliance. Advantages include flexibility and high [[dielectric]] strength. Disadvantages include poor [[bounding]], combustibility (hard to extinguish), and significant smoke development. | |
| − | Silicone | + | Silicone foams have been used in North American as well as the Israeli [[Dimona]] [[nuclear reactor]] buildings, in attempting to [[firestop]] openings within fire-resistance rated wall and floor assemblies, to prevent the spread of flames and smoke from one room to another. The [[Israel]]is switched to the somewhat more expensive yet much safer "[[elastomer]]" version of this product, which avoids most safety concerns associated with the foamed version. |
| − | + | Silicone foam firestops have been the subject of serious controversy and press attention due to lack of proper bounding, smoke development (during the burning of some components in the foam), [[hydrogen]] gas escape, shrinkage, and cracking. These problems were exposed by [[Gerald W. Brown]], leading to a large number of reportable events among licensees (operators of [[nuclear power plant]]s) of the [[Nuclear Regulatory Commission]] (NRC). | |
===Personal care products=== | ===Personal care products=== | ||
Silicones are used as ingredients in some leave-in [[hair conditioner]] products. These formulations utilize silicone's water resistance to prevent humidity from entering a dry hair shaft and ruining the style. | Silicones are used as ingredients in some leave-in [[hair conditioner]] products. These formulations utilize silicone's water resistance to prevent humidity from entering a dry hair shaft and ruining the style. | ||
| + | |||
| + | ===Menstrual cups=== | ||
| + | |||
| + | A [[menstrual cup]] is a type of cup or barrier worn inside the vagina during menstruation to collect menstrual fluid. Menstrual cups are often made of silicone for durability and reusability. | ||
| + | |||
| + | ===Hearing aids=== | ||
| + | |||
| + | Silicone is a common material used in molds for behind-the-ear style [[hearing aids]]. It has excellent sealing properties, making it an ideal choice for patients with profound hearing losses needing high-powered hearing aids. | ||
===Dry cleaning=== | ===Dry cleaning=== | ||
| − | Liquid silicone can be used as a [[dry cleaning]] [[solvent]]. Touted as an "environmentally friendly" alternative to the traditional [[perchloroethylene]] (or perc) solvent, the decamethylpentacyclosiloxane (D5) process has been patented by the company [[GreenEarth Cleaning]]. | + | Liquid silicone can be used as a [[dry cleaning]] [[solvent]]. Touted as an "environmentally friendly" alternative to the traditional [[perchloroethylene]] (or perc) solvent, the decamethylpentacyclosiloxane (D5) process has been patented by the company [[GreenEarth Cleaning]]. The solvent degrades into sand and trace amounts of water and CO2, and waste produced from the D5 dry-cleaning process is nontoxic and nonhazardous. This significantly reduces the environmental impact of a typically high-polluting industry. |
Additionally, liquid silicone is chemically inert, meaning it does not react with fabrics or dyes during the cleaning process. This reduces the amount of fading and shrinking that most dry-cleaned garments experience. | Additionally, liquid silicone is chemically inert, meaning it does not react with fabrics or dyes during the cleaning process. This reduces the amount of fading and shrinking that most dry-cleaned garments experience. | ||
| Line 111: | Line 118: | ||
* [[Sealant]] | * [[Sealant]] | ||
* [[Silicon]] | * [[Silicon]] | ||
| + | |||
| + | == Notes == | ||
| + | <references/> | ||
| + | |||
| + | == References == | ||
| + | |||
| + | * Bondurant, Stuart, Virginia L. Ernster, and Roger Herdman, (eds.). 2000. ''Safety of Silicone Breast Implants''. Washington, DC: Institute of Medicine. ISBN 0585215553. | ||
| + | |||
| + | * Clarson, Stephen J., et al., (eds.). 2007. ''Science and Technology of Silicones and Silicone-Modified Materials''. ACS Symposium Series, 964. Washington, DC: American Chemical Society. ISBN 9780841274372. | ||
| + | |||
| + | * Koerner, G. 1991. ''Silicones: Chemistry and Technology''. Boca Raton: CRC Press. ISBN 0849377404. | ||
| + | |||
| + | * Rochow, Eugene George. 1951. ''An Introduction to the Chemistry of the Silicones''. New York: Wiley. OCLC 58852709. | ||
| + | |||
| + | * Stewart, Mary White. 1998. ''Silicone Spills: Breast Implants on Trial''. Westport, CT: Praeger. ISBN 0275963594. | ||
== External links == | == External links == | ||
| + | All links retrieved September 18, 2015. | ||
| − | + | * [http://www.ontla.on.ca/web/committee-proceedings/committee_transcripts_details.do?locale=en&Date=1997-10-27&ParlCommID=828&BillID=&Business=Hydro+Stakeholders Committee Transcripts: Select Committee on Hydro Nuclear Affairs - October 27, 1997 - Hydro Stakeholders.] – ''Legislative Assembly of Ontario''. | |
| − | * [http://www.ontla.on.ca/web/committee-proceedings/committee_transcripts_details.do?locale=en&Date=1997-10-27&ParlCommID=828&BillID=&Business=Hydro+Stakeholders | + | * [http://www.nirs.org/reactorwatch/mox/nirsmcguirecatawbacontentions.htm NIRS Reactorwatch] – ''U.S. Nuclear Regulatory Commission''. |
| − | Committee Transcripts: Select Committee on Hydro Nuclear Affairs - October 27, 1997 - Hydro Stakeholders.] ''Legislative Assembly of Ontario'' | + | * [http://www.ccnr.org/nucaware_foam_pr.html Flammable 'Firestops' Used in CANDU Reactors.] – Press release of U.S. Representative [[Ed Markey]]'s Statements. |
| − | * [http://www.nirs.org/reactorwatch/mox/nirsmcguirecatawbacontentions.htm NIRS Reactorwatch] ''U.S. Nuclear Regulatory Commission'' | + | * [http://www.nrc.gov/reading-rm/doc-collections/gen-comm/info-notices/1988/in88056.html Potential Problems with Silicone Foam Fire Barrier Penetration Seals.] – ''U.S. Nuclear Regulatory Commission''. |
| − | * [http://www.ccnr.org/nucaware_foam_pr.html Flammable 'Firestops' Used in CANDU Reactors.] Press release of U.S. Representative [[Ed Markey]]'s Statements | + | * [http://www.silicones-science.com/ Silicones Europe.] – ''Centre Européen des Silicones'' (CES). |
| − | * [http://www.nrc.gov/reading-rm/doc-collections/gen-comm/info-notices/1988/in88056.html Potential Problems with Silicone Foam Fire Barrier Penetration Seals.] ''U.S. Nuclear Regulatory Commission'' | + | * [http://www.dowcorning.com/content/sitech/sitechbasics/default.asp The Basics of Silicon Chemistry.] – ''Dow Corning''. |
| − | * [http://www.silicones-science.com/ Silicones | ||
| − | * [http://www.dowcorning.com/content/sitech/sitechbasics/default.asp The Basics of Silicon Chemistry.] ''Dow Corning'' | ||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
| − | |||
| − | {{ | + | |
| + | {{credits|Silicone|152840549|Silicone_rubber|149970868}} | ||
Revision as of 16:47, 18 September 2015
- Not to be confused with the element silicon.
Silicones (more accurately called polymerized siloxanes or polysiloxanes) are mixed inorganic-organic polymers. Their general chemical formula can be written as [R2SiO]n, where R corresponds to an organic group such as methyl, ethyl, or phenyl. By varying their composition and molecular structures, silicones with a range of properties can be prepared. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common siloxane is polydimethylsiloxane (PDMS), a silicone oil. The second largest group of silicone materials is based on silicone resins.
Different types of silicones have been developed for a variety of applications. For instance, they are used as sealants, molds, lubricants, dry cleaning solvents, electrical insulators, and protective material for electronic components. They are also found in some firestops, personal care products, and hearing aids. However, their uses in breast implants and nuclear reactor buildings have stirred controversy.
Chemical structure and terminology
Silicone is often mistakenly referred to as "silicon." Although silicones contain silicon atoms, they are not made up exclusively of silicon, and they have completely different physical characteristics from elemental silicon.
The word "silicone" is derived from ketone. Dimethylsilicone and dimethyl ketone (acetone) have analogous chemical formulas, therefore it was surmised (incorrectly) that they have analogous structures.[1] In the case of an acetone (or any ketone) molecule, there is a double bond between a carbon atom and an oxygen atom. On the other hand, a silicone molecule does not contain a double bond between a silicon atom and an oxygen atom. Chemists have found that the silicon atom forms a single bond with each of two oxygen atoms, rather than a double bond to a single atom.
Polysiloxanes are called "silicones" due to early mistaken assumptions about their structure. They consist of an inorganic silicon-oxygen backbone (...-Si-O-Si-O-Si-O-...) with organic side groups attached to the silicon atoms (see the figure showing the structure of polydimethylsiloxane). In some cases, organic side groups can be used to link two or more of these -Si-O- backbones together.
By varying the -Si-O- chain lengths, side groups, and crosslinking, a variety of silicones can be synthesized. The most common siloxane is linear polydimethylsiloxane (PDMS), a silicone oil (see the structure shown in the figure). The second largest group of silicone materials is based on silicone resins, which are formed by branched and cage-like oligosiloxanes.
Synthesis
Silicones are synthesized from chlorosilanes, tetraethoxysilane, and related compounds. In the case of PDMS, the starting material is dimethylchlorosilane, which reacts with water as follows:
- n [Si(CH3)2Cl2] + n [H2O] → [Si(CH3)2O]n + 2n HCl
During polymerization, this reaction evolves potentially hazardous hydrogen chloride gas. For medical uses, a process was developed where the chlorine atoms in the silane precursor were replaced with acetate groups, so that the reaction product of the final curing process is nontoxic acetic acid (vinegar). As a side effect, the curing process is also much slower in this case. This is the chemistry used in many consumer applications, such as silicone caulk and adhesives.
Silane precursors with more acid-forming groups and fewer methyl groups, such as methyltrichlorosilane, can be used to introduce branches or cross-links in the polymer chain. Ideally, each molecule of such a compound becomes a branch point. This can be used to produce hard silicone resins. Similarly, precursors with three methyl groups can be used to limit molecular weight, since each such molecule has only one reactive site and so forms the end of a siloxane chain.
Modern silicone resins are made with tetraethoxysilane, which reacts in a milder and more controllable manner than chlorosilanes.
Properties
Some of the most useful properties of silicone include:
- Thermal stability (Constancy of properties over a wide operating range of –100 to 250°C)
- The ability to repel water and form watertight seals
- Excellent resistance to oxygen, ozone, and sunlight
- Flexibility
- Electrically insulating or conductive, depending on structure and composition
- Anti-adhesive
- Low chemical reactivity
- Low toxicity
- High gas permeability[2]
Silicone rubber
A flexible, rubbery polysiloxane is known as silicone rubber. It can be extruded into tubes, strips, solid cord, and custom profiles. It offers excellent resistance to extreme temperatures and is highly inert toward most chemicals. Organic rubber, with a carbon-to-carbon backbone, is generally susceptible to ozone, UV, heat, and other aging factors. Silicone rubber, by contrast, can withstand the effects of these agents, making it the material of choice in many extreme environments. Given its inertness, it is used in many medical applications, including medical implants.
Many specialist grades of silicone rubber have these properties: electrical conductivity, low smoke emission, flame retardation, glow in the dark, and resistance to steam, gases, oils, acids, and other chemicals.
Uses of Silicone
Moldmaking material
Two-part silicone systems are used to create rubber molds, which can be used for production casting of resins, foams, rubber, and low-temp alloys. A silicone mold generally requires little or no mold release or surface preparation, as most materials do not adhere to the silicone.
Sealants
One-part silicone sealants are in common use to seal gaps, joints, and crevices in buildings. These silicones cure by absorbing atmospheric moisture. The strength and reliability of silicone rubber is widely acknowledged in the construction industry.
An excellent use of silicone rubber is for automotive sunroof seals, which have to endure harsh temperatures and other environmental conditions such as ozone, UV light, and pollution, not to mention common automotive cleaners, waxes, and so forth.
Lubricant
In the plumbing and automotive fields, silicone grease is often used as a lubricant. In plumbing, the grease is typically applied to O-rings in faucets and valves. In the automotive field, silicone grease is typically used as a lubricant for brake components, as it is stable at high temperatures, is insoluble in water, and far less likely than other lubricants to foul brake pads.
Cooking applications
Silicone is also impregnated into parchment paper and used as a non-stick material for applications such as baking and steaming. The silicone also makes the paper heat- and grease-resistant. This allows the paper to line cookie sheets and act as a replacement for greasing, thereby speeding mass production of baked goods. It is also commonly used in pouch cooking, where ingredients are sealed into a container made of parchment paper and allowed to steam.
Silicone rubber is used to make utensils (notably spatulas) and bakeware.
Silicone resins are used in heat-resistant dishware. These often resemble ceramic items but are much less brittle, making them popular for use with babies.
Electrical and electronic components
Automotive spark plug wires are often insulated by multiple layers of silicone. In addition, electronic components are sometimes protected from environmental influences by enclosing them in silicone. This increases their stability against mechanical shock, radiation, and vibration. Silicones are selected over polyurethane or epoxy encapsulation when a wide operating temperature range is required (−150 to 600°F). Silicones also have the advantage of little heat increase in the curing process, low toxicity, good electrical properties, and high purity. Therefore they are used when durability and high performance are required of components under demanding conditions, such as for satellites in space.
Silicone breast implants
In the 1980s and 1990s, controversy developed around claims that the silicone gel in breast implants was responsible for a number of systemic health problems, including autoimmune diseases and cancer. Multiple lawsuits claiming injury from implants resulted in the 1998 bankruptcy of Dow Corning and a moratorium on the use of silicone implants for breast augmentation in the US and Canada pending study. However, multiple studies and expert review panels performed worldwide since then have consistently concluded that women with silicone breast implants are no more likely to develop systemic illness than women without breast implants. In 2006, both Health Canada and the U.S. Food and Drug Administration (FDA) adopted positions similar to other countries in permitting the use of silicone implants for cosmetic breast augmentation in their respective countries.
Firestops
When properly installed, silicone foam firestops can be fabricated for building code compliance. Advantages include flexibility and high dielectric strength. Disadvantages include poor bounding, combustibility (hard to extinguish), and significant smoke development.
Silicone foams have been used in North American as well as the Israeli Dimona nuclear reactor buildings, in attempting to firestop openings within fire-resistance rated wall and floor assemblies, to prevent the spread of flames and smoke from one room to another. The Israelis switched to the somewhat more expensive yet much safer "elastomer" version of this product, which avoids most safety concerns associated with the foamed version.
Silicone foam firestops have been the subject of serious controversy and press attention due to lack of proper bounding, smoke development (during the burning of some components in the foam), hydrogen gas escape, shrinkage, and cracking. These problems were exposed by Gerald W. Brown, leading to a large number of reportable events among licensees (operators of nuclear power plants) of the Nuclear Regulatory Commission (NRC).
Personal care products
Silicones are used as ingredients in some leave-in hair conditioner products. These formulations utilize silicone's water resistance to prevent humidity from entering a dry hair shaft and ruining the style.
Menstrual cups
A menstrual cup is a type of cup or barrier worn inside the vagina during menstruation to collect menstrual fluid. Menstrual cups are often made of silicone for durability and reusability.
Hearing aids
Silicone is a common material used in molds for behind-the-ear style hearing aids. It has excellent sealing properties, making it an ideal choice for patients with profound hearing losses needing high-powered hearing aids.
Dry cleaning
Liquid silicone can be used as a dry cleaning solvent. Touted as an "environmentally friendly" alternative to the traditional perchloroethylene (or perc) solvent, the decamethylpentacyclosiloxane (D5) process has been patented by the company GreenEarth Cleaning. The solvent degrades into sand and trace amounts of water and CO2, and waste produced from the D5 dry-cleaning process is nontoxic and nonhazardous. This significantly reduces the environmental impact of a typically high-polluting industry.
Additionally, liquid silicone is chemically inert, meaning it does not react with fabrics or dyes during the cleaning process. This reduces the amount of fading and shrinking that most dry-cleaned garments experience.
See also
- Breast implant
- Dental dam
- Dry cleaning
- Firestop
- Nuclear Reactor
- Parchment
- Sealant
- Silicon
Notes
- ↑ The same terminology is used for compounds such as silane (an analog of methane).
- ↑ At room temperature (25°C), the permeability of silicone rubber for gases like oxygen is approximately 400 times that of butyl rubber, making silicone useful for medical applications. At the same time, this property precludes it from applications where gas-tight seals are necessary.
ReferencesISBN links support NWE through referral fees
- Bondurant, Stuart, Virginia L. Ernster, and Roger Herdman, (eds.). 2000. Safety of Silicone Breast Implants. Washington, DC: Institute of Medicine. ISBN 0585215553.
- Clarson, Stephen J., et al., (eds.). 2007. Science and Technology of Silicones and Silicone-Modified Materials. ACS Symposium Series, 964. Washington, DC: American Chemical Society. ISBN 9780841274372.
- Koerner, G. 1991. Silicones: Chemistry and Technology. Boca Raton: CRC Press. ISBN 0849377404.
- Rochow, Eugene George. 1951. An Introduction to the Chemistry of the Silicones. New York: Wiley. OCLC 58852709.
- Stewart, Mary White. 1998. Silicone Spills: Breast Implants on Trial. Westport, CT: Praeger. ISBN 0275963594.
External links
All links retrieved September 18, 2015.
- Committee Transcripts: Select Committee on Hydro Nuclear Affairs - October 27, 1997 - Hydro Stakeholders. – Legislative Assembly of Ontario.
- NIRS Reactorwatch – U.S. Nuclear Regulatory Commission.
- Flammable 'Firestops' Used in CANDU Reactors. – Press release of U.S. Representative Ed Markey's Statements.
- Potential Problems with Silicone Foam Fire Barrier Penetration Seals. – U.S. Nuclear Regulatory Commission.
- Silicones Europe. – Centre Européen des Silicones (CES).
- The Basics of Silicon Chemistry. – Dow Corning.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.