Difference between revisions of "Hydrogen" - New World Encyclopedia
David Burton (talk | contribs) (Add categories) |
Rosie Tanabe (talk | contribs) m |
||
| (18 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{Elementbox_header | number=1 | symbol=H | name=hydrogen | left= | + | {{Copyedited}}{{Images OK}}{{Submitted}}{{Approved}}{{Paid}} |
| + | {{Elementbox_header | number=1 | symbol=H | name=hydrogen | left=(none) | right=[[helium]] | above=- | below=[[lithium|Li]] | color1=#a0ffa0 | color2=green }} | ||
{{Elementbox_series | [[nonmetal]]s }} | {{Elementbox_series | [[nonmetal]]s }} | ||
{{Elementbox_groupperiodblock | group=1 | period=1 | block=s }} | {{Elementbox_groupperiodblock | group=1 | period=1 | block=s }} | ||
{{Elementbox_appearance_img | H,1| colorless }} | {{Elementbox_appearance_img | H,1| colorless }} | ||
| − | {{Elementbox_atomicmass_gpm | [[ | + | {{Elementbox_atomicmass_gpm | 1.00794[[List of elements by atomic mass|(7)]]}} |
{{Elementbox_econfig | 1s<sup>1</sup> }} | {{Elementbox_econfig | 1s<sup>1</sup> }} | ||
{{Elementbox_epershell | 1 }} | {{Elementbox_epershell | 1 }} | ||
| Line 9: | Line 10: | ||
{{Elementbox_phase | [[gas]] }} | {{Elementbox_phase | [[gas]] }} | ||
{{Elementbox_density_gplstp | 0.08988 }} | {{Elementbox_density_gplstp | 0.08988 }} | ||
| − | {{Elementbox_meltingpoint | k=14.01 | c= | + | {{Elementbox_meltingpoint | k=14.01 | c=−259.14 | f=−434.45 }} |
| − | {{Elementbox_boilingpoint | k=20.28 | c= | + | {{Elementbox_boilingpoint | k=20.28 | c=−252.87 | f=−423.17 }} |
| + | |- | ||
| + | | [[Triple point]] || 13.8033 K, 7.042 kPa | ||
| + | {{Elementbox_criticalpoint | k=32.97 | mpa=1.293 }} | ||
{{Elementbox_heatfusion_kjpmol | (H<sub>2</sub>) 0.117 }} | {{Elementbox_heatfusion_kjpmol | (H<sub>2</sub>) 0.117 }} | ||
{{Elementbox_heatvaporiz_kjpmol | (H<sub>2</sub>) 0.904 }} | {{Elementbox_heatvaporiz_kjpmol | (H<sub>2</sub>) 0.904 }} | ||
{{Elementbox_heatcapacity_jpmolkat25 | (H<sub>2</sub>)<br />28.836 }} | {{Elementbox_heatcapacity_jpmolkat25 | (H<sub>2</sub>)<br />28.836 }} | ||
{{Elementbox_vaporpressure_katpa | | | | | 15 | 20 | comment= }} | {{Elementbox_vaporpressure_katpa | | | | | 15 | 20 | comment= }} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{{Elementbox_section_atomicprop | color1=#a0ffa0 | color2=green }} | {{Elementbox_section_atomicprop | color1=#a0ffa0 | color2=green }} | ||
{{Elementbox_crystalstruct | hexagonal }} | {{Elementbox_crystalstruct | hexagonal }} | ||
| − | {{Elementbox_oxistates | '''1''', | + | {{Elementbox_oxistates | '''1''', −1<br />([[amphoteric]] oxide) }} |
{{Elementbox_electroneg_pauling | 2.20 }} | {{Elementbox_electroneg_pauling | 2.20 }} | ||
{{Elementbox_ionizationenergies1 | 1312.0 }} | {{Elementbox_ionizationenergies1 | 1312.0 }} | ||
| − | {{Elementbox_atomicradius_pm | + | {{Elementbox_atomicradius_pm | 25 }} |
| − | {{Elementbox_atomicradiuscalc_pm | + | {{Elementbox_atomicradiuscalc_pm | 53 }} ([[Bohr radius]]) |
| − | {{Elementbox_covalentradius_pm | + | {{Elementbox_covalentradius_pm | 37 }} |
| − | {{Elementbox_vanderwaalsrad_pm | + | {{Elementbox_vanderwaalsrad_pm | 120 }} |
{{Elementbox_section_miscellaneous | color1=#a0ffa0 | color2=green }} | {{Elementbox_section_miscellaneous | color1=#a0ffa0 | color2=green }} | ||
| − | |||
{{Elementbox_thermalcond_wpmkat300k | 180.5 m}} | {{Elementbox_thermalcond_wpmkat300k | 180.5 m}} | ||
{{Elementbox_speedofsound_mps | (gas, 27 °C) 1310 }} | {{Elementbox_speedofsound_mps | (gas, 27 °C) 1310 }} | ||
| − | {{Elementbox_cas_number | 1333-74-0 }} | + | {{Elementbox_cas_number | 1333-74-0 (H<sub>2</sub>)}} |
{{Elementbox_isotopes_begin | isotopesof=hydrogen | color1=#a0ffa0 | color2=green }} | {{Elementbox_isotopes_begin | isotopesof=hydrogen | color1=#a0ffa0 | color2=green }} | ||
{{Elementbox_isotopes_stable | mn=1 | sym=H | na=99.985% | n=0 }} | {{Elementbox_isotopes_stable | mn=1 | sym=H | na=99.985% | n=0 }} | ||
| − | + | |- | |
| − | {{Elementbox_isotopes_decay | mn=3 | sym=H | na=[[Trace radioisotope|trace]] | hl=12.32 | + | | <sup>2</sup>H || 0.0115% || colspan="4" | H is [[stable isotope|stable]] with 1 [[neutron]] |
| + | {{Elementbox_isotopes_decay | mn=3 | sym=H | na=[[Trace radioisotope|trace]] | hl=12.32 years | dm=[[beta minus decay|β<sup>−</sup>]] | de=0.019 | pn=3 | ps=[[helium|He]] }} | ||
{{Elementbox_isotopes_end}} | {{Elementbox_isotopes_end}} | ||
{{Elementbox_footer | color1=#a0ffa0 | color2=green }} | {{Elementbox_footer | color1=#a0ffa0 | color2=green }} | ||
| − | '''Hydrogen''' ([[ | + | '''Hydrogen''' (chemical symbol '''H''', [[atomic number]] 1) is the lightest [[chemical element]] and the most abundant of all elements, constituting roughly 75 percent of the elemental mass of the [[universe]].<ref>[http://imagine.gsfc.nasa.gov/docs/ask_astro/answers/971113i.html Hydrogen in the Universe,] NASA. Retrieved December 27, 2007.</ref> [[Star]]s in the [[main sequence]] are mainly composed of hydrogen in its [[plasma (physics)|plasma]] state. |
| + | |||
| + | In the [[Earth]]'s natural environment, free (uncombined) hydrogen is relatively rare. At [[standard temperature and pressure]], it takes the form of a colorless, odorless, tasteless, highly [[combustion|flammable]] [[gas]] made up of [[diatomic]] molecules (H<sub>2</sub>). On the other hand, the element is widely distributed in combination with other elements, and many of its compounds are vital for living systems. Its most familiar compound is [[water]] (H<sub>2</sub>O). | ||
| + | |||
| + | Elemental hydrogen is industrially produced from [[hydrocarbon]]s such as methane, after which most elemental hydrogen is used "captively" (meaning locally, at the production site). The largest markets are about equally divided between [[fossil fuel]] upgrading (such as [[hydrocracking]]) and [[ammonia]] production (mostly for the [[fertilizer]] market). | ||
| + | {{toc}} | ||
| + | The most common naturally occurring [[isotope]] of hydrogen, known as protium, has a single [[proton]] and no [[neutron]]s. In [[ionic compound]]s, it can take on either a positive charge (becoming a [[Ion|cation]], H<sup>+</sup>, which is a proton) or a negative charge (becoming an anion, H<sup>−</sup>, called a [[hydride]]). It plays a particularly important role in [[acid-base reaction theories|acid-base chemistry]], in which many reactions involve the exchange of protons between soluble molecules. As the only neutral atom for which the [[Schrödinger equation]] can be solved analytically, study of the energetics and bonding of the hydrogen atom has played a key role in the development of [[quantum mechanics]]. | ||
| + | |||
| + | == Etymology == | ||
| + | |||
| + | The term ''hydrogen'' ({{lang-la|''hydrogenium''}}) can be traced to a combination of the [[Greek language|ancient Greek]] words ''hydor'', meaning "water," and ''genes'', meaning "forming." This refers to the observation that when hydrogen burns, it produces water. | ||
| + | |||
| + | ==Natural occurrence== | ||
| + | [[Image:Triangulum.nebula.full.jpg|left|thumb|250px|NGC 604, a giant region of ionized hydrogen in the Triangulum Galaxy]] | ||
| + | Hydrogen is the most abundant element in the universe, making up 75 percent of [[Baryon|normal matter]] by [[mass]] and over 90 percent by number of atoms.<ref>Steve Gagnon, [http://education.jlab.org/itselemental/ele001.html It’s Elemental: Hydrogen,] Jefferson Lab. Retrieved December 27, 2007.</ref> This element is found in great abundance in [[star]]s and [[gas giant]] planets. [[Molecular cloud]]s of H<sub>2</sub> are associated with [[star formation]]. Hydrogen plays a vital role in powering stars through [[proton-proton reaction]] [[nuclear fusion]]. | ||
| + | |||
| + | Throughout the universe, hydrogen is mostly found in the [[atomic]] and [[Plasma (physics)|plasma]] states whose properties are quite different from molecular hydrogen. As a plasma, hydrogen's [[electron]] and [[proton]] are not bound together, resulting in very high electrical [[conductivity]] and high emissivity (producing the light from the [[sun]] and other [[star]]s). The charged particles are highly influenced by [[magnetic field|magnetic]] and [[electric field]]s. For example, in the [[solar wind]] they interact with [[Earth]]'s [[magnetosphere]] giving rise to [[Birkeland current]]s and the [[Aurora (phenomenon)|aurora]]. Hydrogen is found in the neutral atomic state in the [[Interstellar medium]]. The large amount of neutral hydrogen found in the damped Lyman-alpha systems is thought to dominate the cosmological baryonic density of the [[universe]] up to [[redshift]] ''z''=4. | ||
| + | |||
| + | Under ordinary conditions on Earth, elemental hydrogen exists as the diatomic gas, H<sub>2</sub> (for data see table). However, hydrogen gas is very rare in the Earth's atmosphere (1 [[part per million]] by volume) because of its light weight, which enables it to escape Earth's gravity more easily than heavier gases. Although H atoms and H<sub>2</sub> molecules are abundant in interstellar space, they are difficult to generate, concentrate and purify on Earth. Still, hydrogen is the third most abundant element on the Earth's surface.<ref name="ArgonneBasic">[http://www.sc.doe.gov/bes/hydrogen.pdf Basic Research Needs for the Hydrogen Economy,] Argonne National Laboratory, U.S. Department of Energy, Office of Science Laboratory. Retrieved December 27, 2007.</ref> Most of the Earth's hydrogen is in the form of [[chemical compound]]s such as [[hydrocarbon]]s and [[water]].<ref name="Miessler">G. L. Miessler and D. A. Tarr, ''Inorganic Chemistry'', 3rd ed. (Upper Saddle River, NJ: Pearson Prentice Hall, 2004, ISBN 0130354716).</ref> Hydrogen gas is produced by some [[bacteria]] and [[algae]] and is a natural component of [[flatus]]. [[Methane]] is a hydrogen source of increasing importance. | ||
| + | |||
| + | ==History== | ||
| + | ===Discovery of H<sub>2</sub>=== | ||
| + | |||
| + | Hydrogen gas, H<sub>2</sub>, was first artificially produced and formally described by T. Von Hohenheim (also known as [[Paracelsus]], 1493–1541) via the mixing of [[metal]]s with [[strong acid]]s. He was unaware that the flammable [[gas]] produced by this [[chemical reaction]] was a new [[chemical element]]. In 1671, [[Robert Boyle]] rediscovered and described the reaction between [[iron]] filings and dilute [[acid]]s, which results in the production of hydrogen gas.<ref>[http://www.webelements.com/webelements/elements/text/H/hist.html Webelements – Hydrogen Historical Information.] Retrieved December 27, 2007.</ref> | ||
| + | |||
| + | In 1766 [[Henry Cavendish]] was the first to recognize hydrogen gas as a discrete substance, by identifying the gas from a [[metal-acid reaction]] as "inflammable air" and further finding that the gas produces [[water]] when burned. Cavendish had stumbled on hydrogen when experimenting with acids and [[mercury (element)|mercury]]. Although he wrongly assumed that hydrogen was a liberated component of the mercury rather than the acid, he was still able to accurately describe several key properties of hydrogen. He is usually given credit for its discovery as an element. In 1783, [[Antoine Lavoisier]] gave the element the name “hydrogen” when he (with [[Pierre-Simon Laplace]]) reproduced Cavendish's finding that water is produced when hydrogen is burned. Lavoisier's name for the gas won out. | ||
| + | |||
| + | One of the first uses of H<sub>2</sub> was for [[balloon]]s, and later [[airships]]. The H<sub>2</sub> was obtained by reacting [[sulfuric acid]] and metallic [[iron]]. Infamously, H<sub>2</sub> was used in the [[Hindenburg (airship)|Hindenburg]] airship that was destroyed in a midair fire. The highly flammable hydrogen (H<sub>2</sub>) was later replaced for airships and most balloons by the unreactive [[helium]] (He). | ||
| + | |||
| + | ===Role in history of quantum theory=== | ||
| + | |||
| + | Because of its relatively simple atomic structure, consisting only of a proton and an electron, the hydrogen atom, together with the [[spectrum]] of light produced from it or absorbed by it, has been central to the development of the theory of [[atom]]ic structure. Furthermore, the corresponding simplicity of the hydrogen molecule and the corresponding [[cation]] H<sub>2</sub><sup>+</sup> allowed fuller understanding of the nature of the [[chemical bond]], which followed shortly after the quantum mechanical treatment of the hydrogen atom had been developed in the mid-1920s. | ||
| + | |||
| + | One of the first quantum effects to be explicitly noticed (but not understood at the time) was a Maxwell observation involving hydrogen, half a century before full [[Quantum mechanics|quantum mechanical theory]] arrived. Maxwell observed that the [[specific heat capacity]] of H<sub>2</sub> unaccountably departs from that of a diatomic gas below room temperature and begins to increasingly resemble that of a monatomic gas at cryogenic temperatures. According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in H<sub>2</sub> because of its low mass. These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures. Diatomic gases composed of heavier atoms do not have such widely spaced levels and do not exhibit the same effect.<ref name="Berman">R. Berman, A. H. Cooke and R. W. Hill, “Cryogenics,” ''Ann. Rev. Phys. Chem.'' 7 (1956): 1–20.</ref> | ||
| + | |||
| + | ==The hydrogen atom== | ||
| + | ===Electron energy levels=== | ||
| + | [[Image:hydrogen atom.svg|thumb|200px|right|Depiction of a hydrogen atom showing the diameter as about twice the [[Bohr model]] radius (image not to scale)]] | ||
| + | The [[ground state]] [[energy level]] of the electron in a hydrogen atom is 13.6 [[Electronvolt|eV]], which is equivalent to an ultraviolet [[photon]] of roughly 92 nanometers. | ||
| + | |||
| + | The energy levels of hydrogen can be calculated fairly accurately using the [[Bohr model]] of the atom, which conceptualizes the electron as "orbiting" the proton in analogy to [[Earth]]'s orbit of the [[sun]]. However, the [[electromagnetic]] force attracts [[electron]]s and [[proton]]s to one another, while planets and celestial objects are attracted to each other by [[gravity]]. Because of the discretization of [[angular momentum]] postulated in early [[quantum mechanics]] by Bohr, the electron in the Bohr model can only occupy certain allowed distances from the proton, and therefore only certain allowed energies. | ||
| + | |||
| + | A more accurate description of the hydrogen atom comes from a purely quantum mechanical treatment that uses the [[Schrödinger equation]] or the equivalent [[Feynman]] [[path integral formulation]] to calculate the [[probability amplitude|probability density]] of the electron around the proton. Treating the electron as a [[de Broglie hypothesis|matter wave]] reproduces chemical results such as shape of the hydrogen atom more naturally than the particle-based Bohr model, although the energy and spectral results are the same. | ||
| + | |||
| + | Modeling the system fully using the [[reduced mass]] of nucleus and electron (as one would do in the [[two-body problem]] in celestial mechanics) yields an even better formula for the hydrogen spectra, and also the correct spectral shifts for the isotopes [[deuterium]] and [[tritium]]. Very small adjustments in energy levels in the hydrogen atom, which correspond to actual spectral effects, may be determined by using a full quantum mechanical theory which corrects for the effects of [[special relativity]], and by accounting for quantum effects arising from production of virtual particles in the vacuum and as a result of electric fields. | ||
| + | |||
| + | In hydrogen gas, the electronic [[ground state]] [[energy level]] is split into [[hyperfine structure]] levels because of magnetic effects of the quantum mechanical [[spin (physics)|spin]] of the electron and proton. The energy of the atom when the proton and electron spins are aligned is higher than when they are not aligned. The transition between these two states can occur through emission of a photon through a [[magnetic dipole]] transition. [[Radio telescope]]s can detect the radiation produced in this process, which is used to map the distribution of hydrogen in the galaxy. | ||
| + | |||
| + | ===Isotopes=== | ||
| + | [[Image:Hydrogen.svg|thumb|150px|left|Protium, the most common isotope of hydrogen, has one proton and one electron. Unique among all stable isotopes, it has no [[neutrons]].]] | ||
| + | |||
| + | Hydrogen has three naturally occurring isotopes, denoted <sup>1</sup>H, <sup>2</sup>H, and <sup>3</sup>H. Other, highly unstable nuclei (<sup>4</sup>H to <sup>7</sup>H) have been synthesized in the laboratory but not observed in nature.<ref name="Gurov">Y. B. Gurov, D. V. Aleshkin, M. N. Berh, S. V. Lapushkin, et al., Spectroscopy of superheavy hydrogen isotopes in stopped-pion absorption by nuclei, ''Physics of Atomic Nuclei'' 68 (3) (2004): 491–497.</ref><ref name="Korsheninnikov"> A. A. Korsheninnikov, et al., Experimental evidence for the existence of 7H and for a specific structure of 8He, ''Phys. Rev. Lett.'' 90 (2003)” 082501.</ref> | ||
| + | * '''<sup>1</sup>H''' is the most common hydrogen isotope with an abundance of more than 99.98 percent. Because the [[atomic nucleus|nucleus]] of this isotope consists of only a single [[proton]], it is given the descriptive but rarely used formal name ''protium''. | ||
| + | * '''<sup>2</sup>H''', the other stable hydrogen isotope, is known as ''[[deuterium]]'' and contains one proton and one [[neutron]] in its nucleus. Deuterium comprises 0.0026–0.0184 percent (by mole-fraction or atom-fraction) of hydrogen samples on Earth, with the lower number tending to be found in samples of hydrogen gas and the higher enrichments (0.015 percent or 150 parts per million) typical of ocean water. Deuterium is not radioactive, and does not represent a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called [[heavy water]]. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for <sup>1</sup>H-[[NMR spectroscopy]]. Heavy water is used as a [[neutron moderator]] and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial [[nuclear fusion]]. | ||
| + | * '''<sup>3</sup>H''' is known as ''[[tritium]]'' and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into [[Helium]]-3 through [[beta decay]] with a [[half-life]] of 12.32 years.<ref name="Miessler" /> Small amounts of tritium occur naturally because of the interaction of cosmic rays with atmospheric gases; tritium has also been released during [[nuclear weapon]]s tests. It is used in nuclear fusion reactions, as a tracer in [[isotope geochemistry]], and specialized in [[self-powered lighting]] devices. Tritium was once routinely used in chemical and biological labeling experiments as a [[radiolabel]] (this has become less common). | ||
| + | |||
| + | Hydrogen is the only element that has different names for its isotopes in common use today (During the early study of radioactivity, various heavy radioactive isotopes were given names, but such names are no longer used. The symbols D and T (instead of <sup>2</sup>H and <sup>3</sup>H) are sometimes used for deuterium and tritium, but the corresponding symbol P is already in use for [[phosphorus]] and thus is not available for protium. [[IUPAC]] states that while this use is common, it is not preferred. | ||
| + | |||
| + | ==Elemental molecular forms== | ||
| + | [[Image:Liquid hydrogen bubblechamber.jpg|thumb|right|300px|First tracks observed in liquid hydrogen [[bubble chamber]] at the Bevatron]] | ||
| + | |||
| + | There are two different types of diatomic hydrogen molecules that differ by the relative [[spin (physics)|spin]] of their nuclei.<ref>[http://www.uigi.com/hydrogen.html Hydrogen (H<sub>2</sub>) Applications and Uses,] Universal Industrial Gases, Inc. Retrieved December 27, 2007.</ref> In the [[orthohydrogen]] form, the spins of the two protons are parallel and form a triplet state; in the [[parahydrogen]] form the spins are antiparallel and form a singlet. At standard temperature and pressure, hydrogen gas contains about 25 percent of the para form and 75 percent of the ortho form, also known as the "normal form."<ref name="Tikhonov"> V. I. Tikhonov and A. A. Volkov, Separation of water into its ortho and para isomers, ''Science'' 296 (5577) (2002) :2363.</ref> The equilibrium ratio of orthohydrogen to parahydrogen depends on temperature, but since the ortho form is an [[excited state]] and has a higher energy than the para form, it is unstable and cannot be purified. At very low temperatures, the equilibrium state is composed almost exclusively of the para form. The physical properties of pure parahydrogen differ slightly from those of the normal form.<ref name="NASA">[http://smad-ext.grc.nasa.gov/gso/manual/chapter_06.pdf CH. 6 - NASA Glenn Research Center Glenn Safety Manual: Hydrogen,] Document GRC-MQSA.001, March 2006. Retrieved December 27, 2007.</ref> The ortho/para distinction also occurs in other hydrogen-containing molecules or functional groups, such as water and [[methylene]]. | ||
| + | |||
| + | The uncatalyzed interconversion between para and ortho H<sub>2</sub> increases with increasing temperature; thus rapidly condensed H<sub>2</sub> contains large quantities of the high-energy ortho form that convert to the para form very slowly.<ref>Y. Y. Milenko, R. M. Sibileva and M. A. Strzhemechny, Natural ortho-para conversion rate in liquid and gaseous hydrogen, ''J. Low. Temp. Phys.'' 107 (1-2) (1997): 77–92.</ref> The ortho/para ratio in condensed H<sub>2</sub> is an important consideration in the preparation and storage of liquid hydrogen: the conversion from ortho to para is [[exothermic]] and produces enough heat to evaporate the hydrogen liquid, leading to loss of the liquefied material. [[Catalyst]]s for the ortho-para interconversion, such as [[iron]] compounds, are used during hydrogen cooling.<ref name="Svadlenak">R. E. Svadlenak and A. B. Scott, The conversion of ortho-to parahydrogen on iron oxide-zinc oxide catalysts, ''J. Am. Chem. Soc.'' 79(20) (1957): 5385–5388.</ref> | ||
| + | |||
| + | A molecular form called protonated molecular hydrogen, or H<sub>3</sub><sup>+</sup>, is found in the [[interstellar medium]] (ISM), where it is generated by ionization of molecular hydrogen from [[cosmic ray]]s. It has also been observed in the upper atmosphere of the planet [[Jupiter]]. This molecule is relatively stable in the environment of outer space due to the low temperature and density. H<sub>3</sub><sup>+</sup> is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium.<ref>[http://h3plus.uiuc.edu/ H3+ Resource Center.] Universities of Illinois and Chicago. Retrieved December 27, 2007.</ref> | ||
| + | |||
| + | == Properties == | ||
| + | |||
| + | Hydrogen is the lightest element in the [[periodic table]], with an [[atomic mass]] of 1.00794 g/[[mole (unit)|mol]]. For lack of a better place, it is generally shown at the top of group 1 (former group 1A). It is, however, a nonmetal, whereas the other members of group 1 are [[alkali metal]]s. | ||
| + | |||
| + | The [[solubility]] and [[adsorption]] characteristics of hydrogen with various metals are very important in [[metallurgy]] (as many metals can suffer [[hydrogen embrittlement]]) and in developing safe ways to store it for use as a fuel. Hydrogen is highly soluble in many compounds composed of [[rare earth metal]]s and [[transition metal]]s<ref name="Takeshita">T. Takeshita, W. E. Wallace and R. S. Craig, Hydrogen solubility in 1:5 compounds between yttrium or thorium and nickel or cobalt, ''Inorg. Chem.'' 13 (9) (1974): 2282.</ref> and can be dissolved in both [[crystalline]] and [[amorphous solid|amorphous]] metals.<ref name="Kirchheim1">R. Kirchheim, T. Mutschele and W. Kieninger, Hydrogen in amorphous and nanocrystalline metals, ''Mater. Sci. Eng.'' 99 (1988): 457–462.</ref> Hydrogen solubility in metals is influenced by local distortions or impurities in the metal [[crystal lattice]].<ref name="Kirchheim2">R. Kirchheim, Hydrogen solubility and diffusivity in defective and amorphous metals, ''Prog. Mater. Sci.'' 32 (4) (1988): 262–325.</ref> | ||
| + | |||
| + | ===Combustion=== | ||
| + | [[Image:Hindenburg burning.jpg|thumb|250px|right|Hydrogen can combust rapidly in air. It burned rapidly in the ''Hindenburg'' [[airship]] disaster May 6, 1937.]] | ||
| + | |||
| + | Hydrogen gas is highly flammable and will burn at concentrations as low as four percent H<sub>2</sub> in air. The combustion reaction may be written as follows: | ||
| + | :2 H<sub>2</sub>(g) + O<sub>2</sub>(g) → 2 H<sub>2</sub>O(l) + 572 kJ/mol | ||
| + | The reaction generates a large amount of heat. The [[enthalpy]] of combustion is – 286 kJ/mol. | ||
| − | + | When mixed with oxygen across a wide range of proportions, hydrogen explodes upon ignition. Pure hydrogen-oxygen flames are nearly invisible to the naked eye, as illustrated by the faintness of flame from the main [[space shuttle]] engines (as opposed to the easily visible flames from the shuttle boosters). Thus it is difficult to visually detect if a hydrogen leak is burning. | |
| − | = | + | The ''Hindenburg'' airship flames seen in the adjacent picture are hydrogen flames colored with material from the covering skin of the zeppelin which contained carbon and pyrophoric aluminum powder, as well as other combustible materials.<ref name="Bain">A. Bain and W. D. Van Vorst, The Hindenburg tragedy revisited: the fatal flaw exposed, ''International Journal of Hydrogen Energy'' 24 (5) (1999): 399–403.</ref> Regardless of the cause of this fire, this was clearly primarily a hydrogen fire since skin of the airship alone would have taken many hours to burn.<ref>John Dziadecki, [http://spot.colorado.edu/~dziadeck/zf/LZ129fire.htm Hindenburg Hydrogen Fire.] Retrieved December 27, 2007.</ref> Another characteristic of hydrogen fires is that the flames tend to ascend rapidly with the gas in air, as illustrated by the ''Hindenburg'' flames, causing less damage than [[hydrocarbon]] fires. For example, two-thirds of the ''Hindenburg'' passengers survived the hydrogen fire, and many of the deaths that occurred were from falling or from gasoline burns.<ref>[http://www.hydropole.ch/Hydropole/Intro/Hindenburg.htm The Hindenburg Disaster,] Swiss Hydrogen Association. Retrieved December 27, 2007.</ref> |
| − | Hydrogen | ||
| − | + | === Reaction with halogens === | |
| − | H<sub>2</sub> | + | H<sub>2</sub> reacts directly with other oxidizing elements. A violent and spontaneous reaction can occur at room temperature with [[chlorine]] and [[fluorine]], forming the corresponding hydrogen halides: [[hydrogen chloride]] and [[hydrogen fluoride]]. |
| − | + | ==Compounds== | |
| + | ===Covalent and organic compounds=== | ||
| − | + | With the exception of the above-mentioned reactions, H<sub>2</sub> is not very reactive under standard conditions. It does, however, form compounds with most elements. Millions of [[hydrocarbon]]s are known, but they are not formed by the direct reaction of elementary hydrogen and carbon (although [[synthesis gas]] production followed by the [[Fischer-Tropsch process]] to make hydrocarbons comes close to being an exception, as this begins with coal and the elemental hydrogen is generated in situ). Hydrogen can form compounds with elements that are more [[electronegative]], such as [[halogen]]s (e.g., F, Cl, Br, I) and [[chalcogen]]s (O, S, Se); in these compounds hydrogen takes on a partial positive charge. When bonded to [[fluorine]], [[oxygen]], or [[nitrogen]], hydrogen can participate in a form of strong noncovalent bonding called [[hydrogen bond]]ing, which is critical to the stability of many biological molecules. Hydrogen also forms compounds with less electronegative elements, such as the [[metal]]s and [[metalloid]]s, in which it takes on a partial negative charge. These compounds are often known as [[hydride]]s. | |
| − | + | ||
| − | + | Hydrogen forms a vast array of compounds with [[carbon]]. Because of their general association with living things, these compounds came to be called [[organic compound]]s; the study of their properties is known as [[organic chemistry]] and their study in the context of living [[organism]]s is known as [[biochemistry]]. By some definitions, "organic" compounds are only required to contain carbon, but most of them also contain hydrogen, and the carbon-hydrogen bond is responsible for many of their chemical characteristics. | |
| − | + | ||
| − | + | In [[inorganic chemistry]], hydrides can also serve as [[bridging ligand]]s that link two metal centers in a [[coordination complex]]. This function is particularly common in [[group 13 element]]s, especially in [[borane]]s ([[boron]] hydrides) and [[aluminum]] complexes, as well as in clustered [[carborane]]s.<ref name="Miessler" /> | |
| − | + | ||
| − | + | ===Hydrides=== | |
| − | + | ||
| − | + | Compounds of hydrogen are often called [[hydride]]s, a term that is used fairly loosely. To chemists, the term "hydride" usually implies that the H atom has acquired a negative or anionic character, denoted H<sup>−</sup>. The existence of the hydride anion, suggested by G. N. Lewis in 1916 for group I and II salt-like hydrides, was demonstrated by Moers in 1920 with the electrolysis of molten [[lithium hydride]] (LiH), that produced a [[stoichiometric]] quantity of hydrogen at the anode.<ref name="Moers">K. Moers,. 2. ''Z. Anorg. Allgem. Chem.'' 113 (1920): 191.</ref> For hydrides other than group I and II metals, the term is quite misleading, considering the low electronegativity of hydrogen. An exception in group II hydrides is BeH<sub>2</sub>, which is polymeric. In [[lithium aluminum hydride]], the AlH<sub>4</sub><sup>−</sup> anion carries hydridic centers firmly attached to the Al(III). Although hydrides can be formed with almost all main-group elements, the number and combination of possible compounds varies widely; for example, there are over one hundred binary borane hydrides known, but only one binary aluminum hydride.<ref name="Downs">A. J. Downs and C. R. Pulham, The hydrides of aluminium, gallium, indium, and thallium: a re-evaluation, ''Chem. Soc. Rev.'' 23 (1994): 175–183.</ref> Binary [[indium]] hydride has not yet been identified, although larger complexes exist.<ref name="Hibbs">D. E. Hibbs, C. Jones and N. A. Smithies, A remarkably stable indium trihydride complex: Synthesis and characterization of [InH3{P(C6H11)3}], ''Chem. Commum.'' (1999): 185–186.</ref> | |
| + | |||
| + | ==="Protons" and acids=== | ||
| + | |||
| + | Oxidation of H<sub>2</sub> formally gives the [[proton]], H<sup>+</sup>. This species is central to discussion of [[acid]]s, though the term proton is used loosely to refer to positively charged or [[cation]]ic hydrogen, denoted H<sup>+</sup>. A bare proton H<sup>+</sup> cannot exist in solution because of its strong tendency to attach itself to atoms or molecules with electrons. To avoid the convenient fiction of the naked "solvated proton" in solution, acidic aqueous solutions are sometimes considered to contain the [[hydronium]] ion (H<sub>3</sub>O<sup>+</sup>) organized into clusters to form H<sub>9</sub>O<sub>4</sub><sup>+</sup>.<ref name="Okumura">M. Okumura, L. I. Yeh, J. D. Myers and Y. T. Lee, Infrared spectra of the solvated hydronium ion: Vibrational predissociation spectroscopy of mass-selected H3O+•H2On•H2m (1990).</ref> Other [[oxonium]] ions are found when water is in solution with other solvents. | ||
| − | + | Although exotic on earth, one of the most common ions in the universe is the [[Protonated molecular hydrogen|H<sub>3</sub><sup>+</sup>]] ion, known as protonated molecular hydrogen or the triatomic hydrogen cation.<ref name="Carrington">A. Carrington and I. R. McNab, The infrared predissociation spectrum of triatomic hydrogen cation (H3+), ''Accounts of Chemical Research'' 22 (1989): 218–222.</ref> | |
| − | == | + | ==Production== |
| − | |||
| − | < | + | H<sub>2</sub> is produced in chemistry and biology laboratories, often as a byproduct of other reactions; in industry for the [[hydrogenation]] of unsaturated substrates; and in nature as a means of expelling [[redox|reducing]] equivalents in biochemical reactions. |
| − | == | + | ===Laboratory syntheses=== |
| − | |||
| − | + | In the [[laboratory]], H<sub>2</sub> is usually prepared by the reaction of acids on metals such as [[zinc]]. | |
| + | :[[zinc|Zn]] + 2 H<sup>+</sup> → Zn<sup>2+</sup> + H<sub>2</sub> | ||
| − | + | [[Aluminum]] produces H<sub>2</sub> upon treatment with an acid or a base: | |
| − | + | :2 Al + 6 H<sub>2</sub>O → 2 Al(OH)<sub>3</sub> + 3 H<sub>2</sub> | |
| − | + | The [[electrolysis]] of water is a simple method of producing hydrogen, although the resulting hydrogen necessarily has less energy content than was required to produce it. A low-voltage current is run through the water, and gaseous oxygen forms at the [[anode]] while gaseous hydrogen forms at the [[cathode]]. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals (iron, for instance, would oxidize, and thus decrease the amount of oxygen given off). The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is between 80 and 94 percent.<ref>[http://bellona.org/filearchive/fil_Hydrogen_6-2002.pdf Bellona Report on Hydrogen.] Retrieved December 27, 2007.</ref> | |
| + | :2H<sub>2</sub>O(aq) → 2H<sub>2</sub>(g) + O<sub>2</sub>(g) | ||
| − | + | In 2007 it was discovered that an alloy of [[aluminum]] and [[gallium]] in pellet form added to water could be used to generate hydrogen.<ref>[http://www.physorg.com/news98556080.html “New process generates hydrogen from aluminum alloy to run engines, fuel cells,”] Physorg.com (May 16, 2007). Retrieved December 27, 2007.</ref> The process creates also creates alumina, but the expensive gallium, which prevents the formation of an oxide skin on the pellets, can be reused. This potentially has important implications for a hydrogen economy, since hydrogen can be produced on-site and does not need to be transported. | |
| − | + | ===Industrial syntheses=== | |
| + | Hydrogen can be prepared in several different ways but the economically most important processes involve removal of hydrogen from hydrocarbons. Commercial bulk hydrogen is usually produced by the [[steam reforming]] of [[natural gas]].<ref name="Oxtoby">D. W. Oxtoby, H. P. Gillis and N. H. Nachtrieb, ''Principles of Modern Chemistry'', 5th ed. (Belmont, CA: Thomson Brooks/Cole, 2002, ISBN 0030353734).</ref> At high temperatures (700–1100 °C; 1,300–2,000 °F), steam (water vapor) reacts with methane to yield [[carbon monoxide]] and H<sub>2</sub>. | ||
:[[methane|CH<sub>4</sub>]] + [[water|H<sub>2</sub>O]] → [[carbon monoxide|CO]] + 3 H<sub>2</sub> | :[[methane|CH<sub>4</sub>]] + [[water|H<sub>2</sub>O]] → [[carbon monoxide|CO]] + 3 H<sub>2</sub> | ||
| − | + | This reaction is favored at low pressures but is nonetheless conducted at high pressures (20 atm; 600 inHg) since high pressure H<sub>2</sub> is the most marketable product. The product mixture is known as "[[synthesis gas]]" because it is often used directly for the production of [[methanol]] and related compounds. [[Hydrocarbon]]s other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon: | |
| + | :[[methane|CH<sub>4</sub>]] → C + 2 H<sub>2</sub> | ||
| + | Consequently, steam reforming typically employs an excess of H<sub>2</sub>O. | ||
| + | Additional hydrogen from steam reforming can be recovered from the carbon monoxide through the [[water gas shift reaction]], especially with an [[iron oxide]] catalyst. This reaction is also a common industrial source of [[carbon dioxide]]:<ref name="Oxtoby" /> | ||
:[[carbon monoxide|CO]] + [[water|H<sub>2</sub>O]] → [[carbon dioxide|CO<sub>2</sub>]] + H<sub>2</sub> | :[[carbon monoxide|CO]] + [[water|H<sub>2</sub>O]] → [[carbon dioxide|CO<sub>2</sub>]] + H<sub>2</sub> | ||
| − | + | Other important methods for H<sub>2</sub> production include partial oxidation of hydrocarbons: | |
| − | + | :[[methane|CH<sub>4</sub>]] + 0.5 [[oxygen|O<sub>2</sub>]] → [[carbon monoxide|CO]] + 2 H<sub>2</sub> | |
| − | + | and the coal reaction, which can serve as a prelude to the shift reaction above:<ref name="Oxtoby" /> | |
| + | :[[carbon|C]] + [[water|H<sub>2</sub>O]] → [[carbon monoxide|CO]] + H<sub>2</sub> | ||
| − | [[ | + | Hydrogen is sometimes produced and consumed in the same industrial process, without being separated. In the [[Haber process]] for the production of [[ammonia]] (the world's fifth-most produced industrial compound), hydrogen is generated from natural gas. |
| − | + | Hydrogen is also produced in usable quantities as a co-product of the major petrochemical processes of [[steam cracking]] and [[reforming]]. [[Electrolysis]] of [[brine]] to yield [[chlorine]] also produces hydrogen as a co-product. | |
| − | |||
| − | [[ | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | ===Biological syntheses=== | |
| − | + | H<sub>2</sub> is a product of some types of [[Fermentation (biochemistry)|anaerobic metabolism]] and is produced by several [[microorganism]]s, usually via reactions [[catalysis|catalyzed]] by [[iron]]- or [[nickel]]-containing [[enzyme]]s called [[hydrogenase]]s. These enzymes catalyze the reversible [[redox]] reaction between H<sub>2</sub> and its component two protons and two electrons. Evolution of hydrogen gas occurs in the transfer of reducing equivalents produced during [[pyruvate]] fermentation to water.<ref> R. Cammack, M. Frey and R. Robson, ''Hydrogen as a Fuel: Learning from Nature'' (London: Taylor & Francis, 2001).</ref> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | [[Water splitting]], in which water is decomposed into its component protons, electrons, and oxygen, occurs in the [[light reaction]]s in all [[photosynthetic]] organisms. Some such organisms—including the [[algae|alga]] ''[[Chlamydomonas reinhardtii]]'' and [[cyanobacteria]]—have evolved a second step in the [[dark reaction]]s in which protons and electrons are reduced to form H<sub>2</sub> gas by specialized hydrogenases in the [[chloroplast]].<ref>O. Kruse, J. Rupprecht, K. P. Bader, S. Thomas-Hal, P. M. Schenk, G. Finazzi and B. Hankamer, Improved photobiological H2 production in engineered green algal cells, ''J. Biol. Chem.'' 280 (40) (2005): 34170–34177.</ref> Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to efficiently synthesize H<sub>2</sub> gas even in the presence of oxygen.<ref>H.O. Smith and Q. Xu, [http://www.hydrogen.energy.gov/pdfs/progress05/iv_e_6_smith.pdf Hydrogen from Water in a Novel Recombinant Oxygen-Tolerant Cyanobacteria System,] United States Department of Energy FY2005 Progress Report, IV.E.6. Retrieved December 27, 2007.</ref> | |
| − | * [[ | + | Other rarer but mechanistically interesting routes to H<sub>2</sub> production also exist in nature. [[Nitrogenase]] produces approximately one equivalent of H<sub>2</sub> for each equivalent of N<sub>2</sub> reduced to ammonia. Some phosphatases reduce [[phosphite]] to H<sub>2</sub>. |
| + | |||
| + | ==Applications== | ||
| + | |||
| + | Large quantities of H<sub>2</sub> are needed in the petroleum and chemical industries. The largest application of H<sub>2</sub> is for the processing ("upgrading") of fossil fuels, and in the production of ammonia. The key consumers of H<sub>2</sub> in the petrochemical plant include [[hydrodealkylation]], [[hydrodesulfurization]], and [[cracking (chemistry)#Hydrocracking|hydrocracking]].<ref>[http://periodic.lanl.gov/elements/1.html Hydrogen,] Los Alamos National Laboratory. Retrieved December 27, 2007.</ref> H<sub>2</sub> has several other important uses. H<sub>2</sub> is used as a hydrogenating agent, particularly in increasing the level of saturation of unsaturated [[fat]]s and [[Vegetable oil|oil]]s (found in items such as [[margarine]]), and in the production of [[methanol]]. It is similarly the source of hydrogen in the manufacture of [[hydrochloric acid]]. H<sub>2</sub> is also used as a [[reducing agent]] of metallic [[ore]]s. | ||
| + | |||
| + | Apart from its use as a reactant, H<sub>2</sub> has wide applications in physics and engineering. It is used as a [[shielding gas]] in [[welding]] methods such as [[atomic hydrogen welding]]. H<sub>2</sub> is used as the rotor coolant in [[electrical generator]]s at [[power station]]s, because it has the highest [[thermal conductivity]] of any gas. Liquid H<sub>2</sub> is used in [[cryogenic]] research, including [[superconductivity]] studies. Since H<sub>2</sub> is [[lighter than air]], having a little more than 1/15th of the density of air, it was once widely used as a lifting agent in [[balloon (aircraft)|balloon]]s and [[airship]]s. However, this use was curtailed after the ''Hindenburg'' disaster convinced the public that the gas was too dangerous for this purpose. Hydrogen is still regularly used for the inflation of [[weather balloon]]s. | ||
| + | |||
| + | Hydrogen's rarer isotopes also each have specific applications. [[Deuterium]] (hydrogen-2) is used in [[CANDU reactor|nuclear fission applications]] as a [[neutron moderator|moderator]] to slow [[neutron]]s, and in [[nuclear fusion]] reactions. Deuterium compounds have applications in [[chemistry]] and [[biology]] in studies of reaction [[isotope effect]]s. [[Tritium]] (hydrogen-3), produced in [[nuclear reactor]]s, is used in the production of [[hydrogen bomb]]s, as an isotopic label in the biosciences, and as a [[Beta radiation|radiation]] source in luminous paints. | ||
| + | |||
| + | The [[triple point]] temperature of equilibrium hydrogen is a defining fixed point on the [[International Temperature Scale of 1990]] (ITS-90). | ||
| + | |||
| + | ===Hydrogen as an energy carrier=== | ||
| + | |||
| + | Hydrogen is not an energy source, except in the hypothetical context of commercial [[nuclear fusion]] power plants using [[deuterium]] or [[tritium]], a technology presently far from development. The [[sun]]'s energy comes from nuclear fusion of hydrogen but this process is difficult to achieve on Earth. Elemental hydrogen from solar, biological, or electrical sources costs more in energy to make than is obtained by burning it. Hydrogen may be obtained from [[fossil]] sources (such as [[methane]]) for less energy than required to make it, but these sources are unsustainable, and are also themselves direct energy sources (and are rightly regarded as the basic source of the energy in the hydrogen obtained from them). | ||
| + | |||
| + | Molecular hydrogen has been widely discussed in the context of energy, as a possible carrier of energy on an economy-wide scale. A theoretical advantage of using H<sub>2</sub> as an energy carrier is the localization and concentration of environmentally unwelcome aspects of hydrogen manufacture from fossil fuel energy sources. For example, CO<sub>2</sub> [[CO2 sequestration|sequestration]] followed by [[carbon capture and storage]] could be conducted at the point of H<sub>2</sub> production from [[methane]]. Hydrogen used in transportation would burn cleanly, without carbon emissions. However, the infrastructure costs associated with full conversion to a hydrogen economy would be substantial.<ref>Joseph Romm, ''The Hype About Hydrogen: Fact and Fiction in the Race to Save the Climate'' (New York: Island Press, 2004, ISBN 1559637048).</ref> In addition, the [[energy density]] of both liquid hydrogen and hydrogen gas at any practicable pressure is significantly less than that of traditional fuel sources. | ||
| + | ==See also== | ||
| + | |||
| + | * [[Biofuel]] | ||
* [[Deuterium]] | * [[Deuterium]] | ||
| + | * [[Electric vehicle]] | ||
| + | * [[Electrolysis]] | ||
* [[Fuel cell]] | * [[Fuel cell]] | ||
| − | * [[ | + | * [[Hydrocarbon]] |
| − | * [[Nuclear | + | * [[Nuclear weapon]] |
| − | * [[Hydrogen | + | * [[Hydrogen vehicle]] |
| − | + | * [[Natural gas]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * [[ | ||
* [[Tritium]] | * [[Tritium]] | ||
| + | * [[Water]] | ||
| + | |||
| + | == Notes == | ||
| + | {{reflist|2}} | ||
==References== | ==References== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * General Electric. 1989. [http://chartofthenuclides.com/default.html Chart of the Nuclides.] General Electric Company. Retrieved December 27, 2007. |
| − | + | * Ferreira-Aparicio, P., M. J. Benito, and J. L. Sanz. 2005. New Trends in Reforming Technologies: from Hydrogen Industrial Plants to Multifuel Microreformers. ''Catalysis Reviews'' 47: 491–588. | |
| − | + | * Krebs, Robert E. 1998. ''The History and Use of Our Earth's Chemical Elements: A Reference Guide.'' Westport, CT: Greenwood Press. ISBN 0313301239 | |
| − | + | * Newton, David E. 1994. ''The Chemical Elements''. New York: Franklin Watts. ISBN 0531125017 | |
| − | * | + | * Rigden, John S. 2002. ''Hydrogen: The Essential Element''. Cambridge, MA: Harvard University Press. ISBN 0531125017 |
| − | * | + | * Romm, Joseph J. 2004. ''The Hype about Hydrogen, Fact and Fiction in the Race to Save the Climate''. Washington, D.C.: Island Press. ISBN 155963703X |
| − | * | + | * Stwertka, Albert. 2002. ''A Guide to the Elements''. New York: Oxford University Press. ISBN 0195150279 |
| − | * | ||
==External links== | ==External links== | ||
| − | + | All links retrieved March 29, 2014. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[ | + | *[http://www.popularmechanics.com/technology/industry/4199381.html “The Truth About Hydrogen”] by Jeff Wise, ''Popular Mechanics'' (November 2006) |
| − | [[ | + | *[http://www.physics.drexel.edu/~tim/open/hydrofin/ The Legendre and Laguerre Polynomials & the Elementary Quantum Mechanical Model of the Hydrogen Atom] by Timothy Jones |
| − | [ | + | *[http://www.greencarcongress.com/biohydrogen/index.html Biohydrogen] – Green Car Congress |
| + | *[http://www.fchea.org/ Fuel Cell and Hydrogen Energy Association] | ||
| + | *[http://hyperphysics.phy-astr.gsu.edu/Hbase/quantum/hydwf.html#c3 Hydrogen Wavefunctions] – HyperPhysics | ||
| + | *[http://www.physorg.com/pdf6381.pdf “Zinc Powder Will Drive your Hydrogen Car”] – Physorg.com | ||
| + | *[http://www3.imperial.ac.uk/newsandeventspggrp/imperialcollege/newssummary/news_1-12-2006-11-4-23?newsid=3016 “Genetically engineered blood protein can be used to produce hydrogen gas from water”] – Imperial College, London | ||
| − | + | {{E number infobox 930-949}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | {{ | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
| − | {{ | + | [[Category:Chemical elements]] |
| + | |||
| + | {{credit|147234836}} | ||
Revision as of 15:31, 29 March 2014
| |||||||||||||||||||||||||
| General | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | hydrogen, H, 1 | ||||||||||||||||||||||||
| Chemical series | nonmetals | ||||||||||||||||||||||||
| Group, Period, Block | 1, 1, s | ||||||||||||||||||||||||
| Appearance | colorless 
| ||||||||||||||||||||||||
| Atomic mass | 1.00794(7) g/mol | ||||||||||||||||||||||||
| Electron configuration | 1s1 | ||||||||||||||||||||||||
| Electrons per shell | 1 | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| Phase | gas | ||||||||||||||||||||||||
| Density | (0 °C, 101.325 kPa) 0.08988 g/L | ||||||||||||||||||||||||
| Melting point | 14.01 K (−259.14 °C, −434.45 °F) | ||||||||||||||||||||||||
| Boiling point | 20.28 K (−252.87 °C, −423.17 °F) | ||||||||||||||||||||||||
| Triple point | 13.8033 K, 7.042 kPa | ||||||||||||||||||||||||
| Critical point | 32.97 K, 1.293 MPa | ||||||||||||||||||||||||
| Heat of fusion | (H2) 0.117 kJ/mol | ||||||||||||||||||||||||
| Heat of vaporization | (H2) 0.904 kJ/mol | ||||||||||||||||||||||||
| Heat capacity | (25 °C) (H2) 28.836 J/(mol·K) | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Crystal structure | hexagonal | ||||||||||||||||||||||||
| Oxidation states | 1, −1 (amphoteric oxide) | ||||||||||||||||||||||||
| Electronegativity | 2.20 (Pauling scale) | ||||||||||||||||||||||||
| Ionization energies | 1st: 1312.0 kJ/mol | ||||||||||||||||||||||||
| Atomic radius | 25 pm | ||||||||||||||||||||||||
| Atomic radius (calc.) | 53 pm (Bohr radius) | ||||||||||||||||||||||||
| Covalent radius | 37 pm | ||||||||||||||||||||||||
| Van der Waals radius | 120 pm | ||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||
| Thermal conductivity | (300 K) 180.5 mW/(m·K) | ||||||||||||||||||||||||
| Speed of sound | (gas, 27 °C) 1310 m/s | ||||||||||||||||||||||||
| CAS registry number | 1333-74-0 (H2) | ||||||||||||||||||||||||
| Notable isotopes | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Hydrogen (chemical symbol H, atomic number 1) is the lightest chemical element and the most abundant of all elements, constituting roughly 75 percent of the elemental mass of the universe.[1] Stars in the main sequence are mainly composed of hydrogen in its plasma state.
In the Earth's natural environment, free (uncombined) hydrogen is relatively rare. At standard temperature and pressure, it takes the form of a colorless, odorless, tasteless, highly flammable gas made up of diatomic molecules (H2). On the other hand, the element is widely distributed in combination with other elements, and many of its compounds are vital for living systems. Its most familiar compound is water (H2O).
Elemental hydrogen is industrially produced from hydrocarbons such as methane, after which most elemental hydrogen is used "captively" (meaning locally, at the production site). The largest markets are about equally divided between fossil fuel upgrading (such as hydrocracking) and ammonia production (mostly for the fertilizer market).
The most common naturally occurring isotope of hydrogen, known as protium, has a single proton and no neutrons. In ionic compounds, it can take on either a positive charge (becoming a cation, H+, which is a proton) or a negative charge (becoming an anion, H−, called a hydride). It plays a particularly important role in acid-base chemistry, in which many reactions involve the exchange of protons between soluble molecules. As the only neutral atom for which the Schrödinger equation can be solved analytically, study of the energetics and bonding of the hydrogen atom has played a key role in the development of quantum mechanics.
Etymology
The term hydrogen (Latin: 'hydrogenium') can be traced to a combination of the ancient Greek words hydor, meaning "water," and genes, meaning "forming." This refers to the observation that when hydrogen burns, it produces water.
Natural occurrence
Hydrogen is the most abundant element in the universe, making up 75 percent of normal matter by mass and over 90 percent by number of atoms.[2] This element is found in great abundance in stars and gas giant planets. Molecular clouds of H2 are associated with star formation. Hydrogen plays a vital role in powering stars through proton-proton reaction nuclear fusion.
Throughout the universe, hydrogen is mostly found in the atomic and plasma states whose properties are quite different from molecular hydrogen. As a plasma, hydrogen's electron and proton are not bound together, resulting in very high electrical conductivity and high emissivity (producing the light from the sun and other stars). The charged particles are highly influenced by magnetic and electric fields. For example, in the solar wind they interact with Earth's magnetosphere giving rise to Birkeland currents and the aurora. Hydrogen is found in the neutral atomic state in the Interstellar medium. The large amount of neutral hydrogen found in the damped Lyman-alpha systems is thought to dominate the cosmological baryonic density of the universe up to redshift z=4.
Under ordinary conditions on Earth, elemental hydrogen exists as the diatomic gas, H2 (for data see table). However, hydrogen gas is very rare in the Earth's atmosphere (1 part per million by volume) because of its light weight, which enables it to escape Earth's gravity more easily than heavier gases. Although H atoms and H2 molecules are abundant in interstellar space, they are difficult to generate, concentrate and purify on Earth. Still, hydrogen is the third most abundant element on the Earth's surface.[3] Most of the Earth's hydrogen is in the form of chemical compounds such as hydrocarbons and water.[4] Hydrogen gas is produced by some bacteria and algae and is a natural component of flatus. Methane is a hydrogen source of increasing importance.
History
Discovery of H2
Hydrogen gas, H2, was first artificially produced and formally described by T. Von Hohenheim (also known as Paracelsus, 1493–1541) via the mixing of metals with strong acids. He was unaware that the flammable gas produced by this chemical reaction was a new chemical element. In 1671, Robert Boyle rediscovered and described the reaction between iron filings and dilute acids, which results in the production of hydrogen gas.[5]
In 1766 Henry Cavendish was the first to recognize hydrogen gas as a discrete substance, by identifying the gas from a metal-acid reaction as "inflammable air" and further finding that the gas produces water when burned. Cavendish had stumbled on hydrogen when experimenting with acids and mercury. Although he wrongly assumed that hydrogen was a liberated component of the mercury rather than the acid, he was still able to accurately describe several key properties of hydrogen. He is usually given credit for its discovery as an element. In 1783, Antoine Lavoisier gave the element the name “hydrogen” when he (with Pierre-Simon Laplace) reproduced Cavendish's finding that water is produced when hydrogen is burned. Lavoisier's name for the gas won out.
One of the first uses of H2 was for balloons, and later airships. The H2 was obtained by reacting sulfuric acid and metallic iron. Infamously, H2 was used in the Hindenburg airship that was destroyed in a midair fire. The highly flammable hydrogen (H2) was later replaced for airships and most balloons by the unreactive helium (He).
Role in history of quantum theory
Because of its relatively simple atomic structure, consisting only of a proton and an electron, the hydrogen atom, together with the spectrum of light produced from it or absorbed by it, has been central to the development of the theory of atomic structure. Furthermore, the corresponding simplicity of the hydrogen molecule and the corresponding cation H2+ allowed fuller understanding of the nature of the chemical bond, which followed shortly after the quantum mechanical treatment of the hydrogen atom had been developed in the mid-1920s.
One of the first quantum effects to be explicitly noticed (but not understood at the time) was a Maxwell observation involving hydrogen, half a century before full quantum mechanical theory arrived. Maxwell observed that the specific heat capacity of H2 unaccountably departs from that of a diatomic gas below room temperature and begins to increasingly resemble that of a monatomic gas at cryogenic temperatures. According to quantum theory, this behavior arises from the spacing of the (quantized) rotational energy levels, which are particularly wide-spaced in H2 because of its low mass. These widely spaced levels inhibit equal partition of heat energy into rotational motion in hydrogen at low temperatures. Diatomic gases composed of heavier atoms do not have such widely spaced levels and do not exhibit the same effect.[6]
The hydrogen atom
Electron energy levels
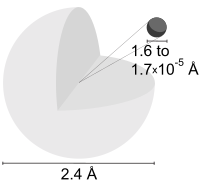
The ground state energy level of the electron in a hydrogen atom is 13.6 eV, which is equivalent to an ultraviolet photon of roughly 92 nanometers.
The energy levels of hydrogen can be calculated fairly accurately using the Bohr model of the atom, which conceptualizes the electron as "orbiting" the proton in analogy to Earth's orbit of the sun. However, the electromagnetic force attracts electrons and protons to one another, while planets and celestial objects are attracted to each other by gravity. Because of the discretization of angular momentum postulated in early quantum mechanics by Bohr, the electron in the Bohr model can only occupy certain allowed distances from the proton, and therefore only certain allowed energies.
A more accurate description of the hydrogen atom comes from a purely quantum mechanical treatment that uses the Schrödinger equation or the equivalent Feynman path integral formulation to calculate the probability density of the electron around the proton. Treating the electron as a matter wave reproduces chemical results such as shape of the hydrogen atom more naturally than the particle-based Bohr model, although the energy and spectral results are the same.
Modeling the system fully using the reduced mass of nucleus and electron (as one would do in the two-body problem in celestial mechanics) yields an even better formula for the hydrogen spectra, and also the correct spectral shifts for the isotopes deuterium and tritium. Very small adjustments in energy levels in the hydrogen atom, which correspond to actual spectral effects, may be determined by using a full quantum mechanical theory which corrects for the effects of special relativity, and by accounting for quantum effects arising from production of virtual particles in the vacuum and as a result of electric fields.
In hydrogen gas, the electronic ground state energy level is split into hyperfine structure levels because of magnetic effects of the quantum mechanical spin of the electron and proton. The energy of the atom when the proton and electron spins are aligned is higher than when they are not aligned. The transition between these two states can occur through emission of a photon through a magnetic dipole transition. Radio telescopes can detect the radiation produced in this process, which is used to map the distribution of hydrogen in the galaxy.
Isotopes

Hydrogen has three naturally occurring isotopes, denoted 1H, 2H, and 3H. Other, highly unstable nuclei (4H to 7H) have been synthesized in the laboratory but not observed in nature.[7][8]
- 1H is the most common hydrogen isotope with an abundance of more than 99.98 percent. Because the nucleus of this isotope consists of only a single proton, it is given the descriptive but rarely used formal name protium.
- 2H, the other stable hydrogen isotope, is known as deuterium and contains one proton and one neutron in its nucleus. Deuterium comprises 0.0026–0.0184 percent (by mole-fraction or atom-fraction) of hydrogen samples on Earth, with the lower number tending to be found in samples of hydrogen gas and the higher enrichments (0.015 percent or 150 parts per million) typical of ocean water. Deuterium is not radioactive, and does not represent a significant toxicity hazard. Water enriched in molecules that include deuterium instead of normal hydrogen is called heavy water. Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for 1H-NMR spectroscopy. Heavy water is used as a neutron moderator and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial nuclear fusion.
- 3H is known as tritium and contains one proton and two neutrons in its nucleus. It is radioactive, decaying into Helium-3 through beta decay with a half-life of 12.32 years.[4] Small amounts of tritium occur naturally because of the interaction of cosmic rays with atmospheric gases; tritium has also been released during nuclear weapons tests. It is used in nuclear fusion reactions, as a tracer in isotope geochemistry, and specialized in self-powered lighting devices. Tritium was once routinely used in chemical and biological labeling experiments as a radiolabel (this has become less common).
Hydrogen is the only element that has different names for its isotopes in common use today (During the early study of radioactivity, various heavy radioactive isotopes were given names, but such names are no longer used. The symbols D and T (instead of 2H and 3H) are sometimes used for deuterium and tritium, but the corresponding symbol P is already in use for phosphorus and thus is not available for protium. IUPAC states that while this use is common, it is not preferred.
Elemental molecular forms
There are two different types of diatomic hydrogen molecules that differ by the relative spin of their nuclei.[9] In the orthohydrogen form, the spins of the two protons are parallel and form a triplet state; in the parahydrogen form the spins are antiparallel and form a singlet. At standard temperature and pressure, hydrogen gas contains about 25 percent of the para form and 75 percent of the ortho form, also known as the "normal form."[10] The equilibrium ratio of orthohydrogen to parahydrogen depends on temperature, but since the ortho form is an excited state and has a higher energy than the para form, it is unstable and cannot be purified. At very low temperatures, the equilibrium state is composed almost exclusively of the para form. The physical properties of pure parahydrogen differ slightly from those of the normal form.[11] The ortho/para distinction also occurs in other hydrogen-containing molecules or functional groups, such as water and methylene.
The uncatalyzed interconversion between para and ortho H2 increases with increasing temperature; thus rapidly condensed H2 contains large quantities of the high-energy ortho form that convert to the para form very slowly.[12] The ortho/para ratio in condensed H2 is an important consideration in the preparation and storage of liquid hydrogen: the conversion from ortho to para is exothermic and produces enough heat to evaporate the hydrogen liquid, leading to loss of the liquefied material. Catalysts for the ortho-para interconversion, such as iron compounds, are used during hydrogen cooling.[13]
A molecular form called protonated molecular hydrogen, or H3+, is found in the interstellar medium (ISM), where it is generated by ionization of molecular hydrogen from cosmic rays. It has also been observed in the upper atmosphere of the planet Jupiter. This molecule is relatively stable in the environment of outer space due to the low temperature and density. H3+ is one of the most abundant ions in the universe, and it plays a notable role in the chemistry of the interstellar medium.[14]
Properties
Hydrogen is the lightest element in the periodic table, with an atomic mass of 1.00794 g/mol. For lack of a better place, it is generally shown at the top of group 1 (former group 1A). It is, however, a nonmetal, whereas the other members of group 1 are alkali metals.
The solubility and adsorption characteristics of hydrogen with various metals are very important in metallurgy (as many metals can suffer hydrogen embrittlement) and in developing safe ways to store it for use as a fuel. Hydrogen is highly soluble in many compounds composed of rare earth metals and transition metals[15] and can be dissolved in both crystalline and amorphous metals.[16] Hydrogen solubility in metals is influenced by local distortions or impurities in the metal crystal lattice.[17]
Combustion

Hydrogen gas is highly flammable and will burn at concentrations as low as four percent H2 in air. The combustion reaction may be written as follows:
- 2 H2(g) + O2(g) → 2 H2O(l) + 572 kJ/mol
The reaction generates a large amount of heat. The enthalpy of combustion is – 286 kJ/mol.
When mixed with oxygen across a wide range of proportions, hydrogen explodes upon ignition. Pure hydrogen-oxygen flames are nearly invisible to the naked eye, as illustrated by the faintness of flame from the main space shuttle engines (as opposed to the easily visible flames from the shuttle boosters). Thus it is difficult to visually detect if a hydrogen leak is burning.
The Hindenburg airship flames seen in the adjacent picture are hydrogen flames colored with material from the covering skin of the zeppelin which contained carbon and pyrophoric aluminum powder, as well as other combustible materials.[18] Regardless of the cause of this fire, this was clearly primarily a hydrogen fire since skin of the airship alone would have taken many hours to burn.[19] Another characteristic of hydrogen fires is that the flames tend to ascend rapidly with the gas in air, as illustrated by the Hindenburg flames, causing less damage than hydrocarbon fires. For example, two-thirds of the Hindenburg passengers survived the hydrogen fire, and many of the deaths that occurred were from falling or from gasoline burns.[20]
Reaction with halogens
H2 reacts directly with other oxidizing elements. A violent and spontaneous reaction can occur at room temperature with chlorine and fluorine, forming the corresponding hydrogen halides: hydrogen chloride and hydrogen fluoride.
Compounds
Covalent and organic compounds
With the exception of the above-mentioned reactions, H2 is not very reactive under standard conditions. It does, however, form compounds with most elements. Millions of hydrocarbons are known, but they are not formed by the direct reaction of elementary hydrogen and carbon (although synthesis gas production followed by the Fischer-Tropsch process to make hydrocarbons comes close to being an exception, as this begins with coal and the elemental hydrogen is generated in situ). Hydrogen can form compounds with elements that are more electronegative, such as halogens (e.g., F, Cl, Br, I) and chalcogens (O, S, Se); in these compounds hydrogen takes on a partial positive charge. When bonded to fluorine, oxygen, or nitrogen, hydrogen can participate in a form of strong noncovalent bonding called hydrogen bonding, which is critical to the stability of many biological molecules. Hydrogen also forms compounds with less electronegative elements, such as the metals and metalloids, in which it takes on a partial negative charge. These compounds are often known as hydrides.
Hydrogen forms a vast array of compounds with carbon. Because of their general association with living things, these compounds came to be called organic compounds; the study of their properties is known as organic chemistry and their study in the context of living organisms is known as biochemistry. By some definitions, "organic" compounds are only required to contain carbon, but most of them also contain hydrogen, and the carbon-hydrogen bond is responsible for many of their chemical characteristics.
In inorganic chemistry, hydrides can also serve as bridging ligands that link two metal centers in a coordination complex. This function is particularly common in group 13 elements, especially in boranes (boron hydrides) and aluminum complexes, as well as in clustered carboranes.[4]
Hydrides
Compounds of hydrogen are often called hydrides, a term that is used fairly loosely. To chemists, the term "hydride" usually implies that the H atom has acquired a negative or anionic character, denoted H−. The existence of the hydride anion, suggested by G. N. Lewis in 1916 for group I and II salt-like hydrides, was demonstrated by Moers in 1920 with the electrolysis of molten lithium hydride (LiH), that produced a stoichiometric quantity of hydrogen at the anode.[21] For hydrides other than group I and II metals, the term is quite misleading, considering the low electronegativity of hydrogen. An exception in group II hydrides is BeH2, which is polymeric. In lithium aluminum hydride, the AlH4− anion carries hydridic centers firmly attached to the Al(III). Although hydrides can be formed with almost all main-group elements, the number and combination of possible compounds varies widely; for example, there are over one hundred binary borane hydrides known, but only one binary aluminum hydride.[22] Binary indium hydride has not yet been identified, although larger complexes exist.[23]
"Protons" and acids
Oxidation of H2 formally gives the proton, H+. This species is central to discussion of acids, though the term proton is used loosely to refer to positively charged or cationic hydrogen, denoted H+. A bare proton H+ cannot exist in solution because of its strong tendency to attach itself to atoms or molecules with electrons. To avoid the convenient fiction of the naked "solvated proton" in solution, acidic aqueous solutions are sometimes considered to contain the hydronium ion (H3O+) organized into clusters to form H9O4+.[24] Other oxonium ions are found when water is in solution with other solvents.
Although exotic on earth, one of the most common ions in the universe is the H3+ ion, known as protonated molecular hydrogen or the triatomic hydrogen cation.[25]
Production
H2 is produced in chemistry and biology laboratories, often as a byproduct of other reactions; in industry for the hydrogenation of unsaturated substrates; and in nature as a means of expelling reducing equivalents in biochemical reactions.
Laboratory syntheses
In the laboratory, H2 is usually prepared by the reaction of acids on metals such as zinc.
- Zn + 2 H+ → Zn2+ + H2
Aluminum produces H2 upon treatment with an acid or a base:
- 2 Al + 6 H2O → 2 Al(OH)3 + 3 H2
The electrolysis of water is a simple method of producing hydrogen, although the resulting hydrogen necessarily has less energy content than was required to produce it. A low-voltage current is run through the water, and gaseous oxygen forms at the anode while gaseous hydrogen forms at the cathode. Typically the cathode is made from platinum or another inert metal when producing hydrogen for storage. If, however, the gas is to be burnt on site, oxygen is desirable to assist the combustion, and so both electrodes would be made from inert metals (iron, for instance, would oxidize, and thus decrease the amount of oxygen given off). The theoretical maximum efficiency (electricity used vs. energetic value of hydrogen produced) is between 80 and 94 percent.[26]
- 2H2O(aq) → 2H2(g) + O2(g)
In 2007 it was discovered that an alloy of aluminum and gallium in pellet form added to water could be used to generate hydrogen.[27] The process creates also creates alumina, but the expensive gallium, which prevents the formation of an oxide skin on the pellets, can be reused. This potentially has important implications for a hydrogen economy, since hydrogen can be produced on-site and does not need to be transported.
Industrial syntheses
Hydrogen can be prepared in several different ways but the economically most important processes involve removal of hydrogen from hydrocarbons. Commercial bulk hydrogen is usually produced by the steam reforming of natural gas.[28] At high temperatures (700–1100 °C; 1,300–2,000 °F), steam (water vapor) reacts with methane to yield carbon monoxide and H2.
This reaction is favored at low pressures but is nonetheless conducted at high pressures (20 atm; 600 inHg) since high pressure H2 is the most marketable product. The product mixture is known as "synthesis gas" because it is often used directly for the production of methanol and related compounds. Hydrocarbons other than methane can be used to produce synthesis gas with varying product ratios. One of the many complications to this highly optimized technology is the formation of coke or carbon:
- CH4 → C + 2 H2
Consequently, steam reforming typically employs an excess of H2O.
Additional hydrogen from steam reforming can be recovered from the carbon monoxide through the water gas shift reaction, especially with an iron oxide catalyst. This reaction is also a common industrial source of carbon dioxide:[28]
Other important methods for H2 production include partial oxidation of hydrocarbons:
and the coal reaction, which can serve as a prelude to the shift reaction above:[28]
Hydrogen is sometimes produced and consumed in the same industrial process, without being separated. In the Haber process for the production of ammonia (the world's fifth-most produced industrial compound), hydrogen is generated from natural gas.
Hydrogen is also produced in usable quantities as a co-product of the major petrochemical processes of steam cracking and reforming. Electrolysis of brine to yield chlorine also produces hydrogen as a co-product.
Biological syntheses
H2 is a product of some types of anaerobic metabolism and is produced by several microorganisms, usually via reactions catalyzed by iron- or nickel-containing enzymes called hydrogenases. These enzymes catalyze the reversible redox reaction between H2 and its component two protons and two electrons. Evolution of hydrogen gas occurs in the transfer of reducing equivalents produced during pyruvate fermentation to water.[29]
Water splitting, in which water is decomposed into its component protons, electrons, and oxygen, occurs in the light reactions in all photosynthetic organisms. Some such organisms—including the alga Chlamydomonas reinhardtii and cyanobacteria—have evolved a second step in the dark reactions in which protons and electrons are reduced to form H2 gas by specialized hydrogenases in the chloroplast.[30] Efforts have been undertaken to genetically modify cyanobacterial hydrogenases to efficiently synthesize H2 gas even in the presence of oxygen.[31]
Other rarer but mechanistically interesting routes to H2 production also exist in nature. Nitrogenase produces approximately one equivalent of H2 for each equivalent of N2 reduced to ammonia. Some phosphatases reduce phosphite to H2.
Applications
Large quantities of H2 are needed in the petroleum and chemical industries. The largest application of H2 is for the processing ("upgrading") of fossil fuels, and in the production of ammonia. The key consumers of H2 in the petrochemical plant include hydrodealkylation, hydrodesulfurization, and hydrocracking.[32] H2 has several other important uses. H2 is used as a hydrogenating agent, particularly in increasing the level of saturation of unsaturated fats and oils (found in items such as margarine), and in the production of methanol. It is similarly the source of hydrogen in the manufacture of hydrochloric acid. H2 is also used as a reducing agent of metallic ores.
Apart from its use as a reactant, H2 has wide applications in physics and engineering. It is used as a shielding gas in welding methods such as atomic hydrogen welding. H2 is used as the rotor coolant in electrical generators at power stations, because it has the highest thermal conductivity of any gas. Liquid H2 is used in cryogenic research, including superconductivity studies. Since H2 is lighter than air, having a little more than 1/15th of the density of air, it was once widely used as a lifting agent in balloons and airships. However, this use was curtailed after the Hindenburg disaster convinced the public that the gas was too dangerous for this purpose. Hydrogen is still regularly used for the inflation of weather balloons.
Hydrogen's rarer isotopes also each have specific applications. Deuterium (hydrogen-2) is used in nuclear fission applications as a moderator to slow neutrons, and in nuclear fusion reactions. Deuterium compounds have applications in chemistry and biology in studies of reaction isotope effects. Tritium (hydrogen-3), produced in nuclear reactors, is used in the production of hydrogen bombs, as an isotopic label in the biosciences, and as a radiation source in luminous paints.
The triple point temperature of equilibrium hydrogen is a defining fixed point on the International Temperature Scale of 1990 (ITS-90).
Hydrogen as an energy carrier
Hydrogen is not an energy source, except in the hypothetical context of commercial nuclear fusion power plants using deuterium or tritium, a technology presently far from development. The sun's energy comes from nuclear fusion of hydrogen but this process is difficult to achieve on Earth. Elemental hydrogen from solar, biological, or electrical sources costs more in energy to make than is obtained by burning it. Hydrogen may be obtained from fossil sources (such as methane) for less energy than required to make it, but these sources are unsustainable, and are also themselves direct energy sources (and are rightly regarded as the basic source of the energy in the hydrogen obtained from them).
Molecular hydrogen has been widely discussed in the context of energy, as a possible carrier of energy on an economy-wide scale. A theoretical advantage of using H2 as an energy carrier is the localization and concentration of environmentally unwelcome aspects of hydrogen manufacture from fossil fuel energy sources. For example, CO2 sequestration followed by carbon capture and storage could be conducted at the point of H2 production from methane. Hydrogen used in transportation would burn cleanly, without carbon emissions. However, the infrastructure costs associated with full conversion to a hydrogen economy would be substantial.[33] In addition, the energy density of both liquid hydrogen and hydrogen gas at any practicable pressure is significantly less than that of traditional fuel sources.
See also
- Biofuel
- Deuterium
- Electric vehicle
- Electrolysis
- Fuel cell
- Hydrocarbon
- Nuclear weapon
- Hydrogen vehicle
- Natural gas
- Tritium
- Water
Notes
- ↑ Hydrogen in the Universe, NASA. Retrieved December 27, 2007.
- ↑ Steve Gagnon, It’s Elemental: Hydrogen, Jefferson Lab. Retrieved December 27, 2007.
- ↑ Basic Research Needs for the Hydrogen Economy, Argonne National Laboratory, U.S. Department of Energy, Office of Science Laboratory. Retrieved December 27, 2007.
- ↑ 4.0 4.1 4.2 G. L. Miessler and D. A. Tarr, Inorganic Chemistry, 3rd ed. (Upper Saddle River, NJ: Pearson Prentice Hall, 2004, ISBN 0130354716).
- ↑ Webelements – Hydrogen Historical Information. Retrieved December 27, 2007.
- ↑ R. Berman, A. H. Cooke and R. W. Hill, “Cryogenics,” Ann. Rev. Phys. Chem. 7 (1956): 1–20.
- ↑ Y. B. Gurov, D. V. Aleshkin, M. N. Berh, S. V. Lapushkin, et al., Spectroscopy of superheavy hydrogen isotopes in stopped-pion absorption by nuclei, Physics of Atomic Nuclei 68 (3) (2004): 491–497.
- ↑ A. A. Korsheninnikov, et al., Experimental evidence for the existence of 7H and for a specific structure of 8He, Phys. Rev. Lett. 90 (2003)” 082501.
- ↑ Hydrogen (H2) Applications and Uses, Universal Industrial Gases, Inc. Retrieved December 27, 2007.
- ↑ V. I. Tikhonov and A. A. Volkov, Separation of water into its ortho and para isomers, Science 296 (5577) (2002) :2363.
- ↑ CH. 6 - NASA Glenn Research Center Glenn Safety Manual: Hydrogen, Document GRC-MQSA.001, March 2006. Retrieved December 27, 2007.
- ↑ Y. Y. Milenko, R. M. Sibileva and M. A. Strzhemechny, Natural ortho-para conversion rate in liquid and gaseous hydrogen, J. Low. Temp. Phys. 107 (1-2) (1997): 77–92.
- ↑ R. E. Svadlenak and A. B. Scott, The conversion of ortho-to parahydrogen on iron oxide-zinc oxide catalysts, J. Am. Chem. Soc. 79(20) (1957): 5385–5388.
- ↑ H3+ Resource Center. Universities of Illinois and Chicago. Retrieved December 27, 2007.
- ↑ T. Takeshita, W. E. Wallace and R. S. Craig, Hydrogen solubility in 1:5 compounds between yttrium or thorium and nickel or cobalt, Inorg. Chem. 13 (9) (1974): 2282.
- ↑ R. Kirchheim, T. Mutschele and W. Kieninger, Hydrogen in amorphous and nanocrystalline metals, Mater. Sci. Eng. 99 (1988): 457–462.
- ↑ R. Kirchheim, Hydrogen solubility and diffusivity in defective and amorphous metals, Prog. Mater. Sci. 32 (4) (1988): 262–325.
- ↑ A. Bain and W. D. Van Vorst, The Hindenburg tragedy revisited: the fatal flaw exposed, International Journal of Hydrogen Energy 24 (5) (1999): 399–403.
- ↑ John Dziadecki, Hindenburg Hydrogen Fire. Retrieved December 27, 2007.
- ↑ The Hindenburg Disaster, Swiss Hydrogen Association. Retrieved December 27, 2007.
- ↑ K. Moers,. 2. Z. Anorg. Allgem. Chem. 113 (1920): 191.
- ↑ A. J. Downs and C. R. Pulham, The hydrides of aluminium, gallium, indium, and thallium: a re-evaluation, Chem. Soc. Rev. 23 (1994): 175–183.
- ↑ D. E. Hibbs, C. Jones and N. A. Smithies, A remarkably stable indium trihydride complex: Synthesis and characterization of [InH3{P(C6H11)3}], Chem. Commum. (1999): 185–186.
- ↑ M. Okumura, L. I. Yeh, J. D. Myers and Y. T. Lee, Infrared spectra of the solvated hydronium ion: Vibrational predissociation spectroscopy of mass-selected H3O+•H2On•H2m (1990).
- ↑ A. Carrington and I. R. McNab, The infrared predissociation spectrum of triatomic hydrogen cation (H3+), Accounts of Chemical Research 22 (1989): 218–222.
- ↑ Bellona Report on Hydrogen. Retrieved December 27, 2007.
- ↑ “New process generates hydrogen from aluminum alloy to run engines, fuel cells,” Physorg.com (May 16, 2007). Retrieved December 27, 2007.
- ↑ 28.0 28.1 28.2 D. W. Oxtoby, H. P. Gillis and N. H. Nachtrieb, Principles of Modern Chemistry, 5th ed. (Belmont, CA: Thomson Brooks/Cole, 2002, ISBN 0030353734).
- ↑ R. Cammack, M. Frey and R. Robson, Hydrogen as a Fuel: Learning from Nature (London: Taylor & Francis, 2001).
- ↑ O. Kruse, J. Rupprecht, K. P. Bader, S. Thomas-Hal, P. M. Schenk, G. Finazzi and B. Hankamer, Improved photobiological H2 production in engineered green algal cells, J. Biol. Chem. 280 (40) (2005): 34170–34177.
- ↑ H.O. Smith and Q. Xu, Hydrogen from Water in a Novel Recombinant Oxygen-Tolerant Cyanobacteria System, United States Department of Energy FY2005 Progress Report, IV.E.6. Retrieved December 27, 2007.
- ↑ Hydrogen, Los Alamos National Laboratory. Retrieved December 27, 2007.
- ↑ Joseph Romm, The Hype About Hydrogen: Fact and Fiction in the Race to Save the Climate (New York: Island Press, 2004, ISBN 1559637048).
ReferencesISBN links support NWE through referral fees
- General Electric. 1989. Chart of the Nuclides. General Electric Company. Retrieved December 27, 2007.
- Ferreira-Aparicio, P., M. J. Benito, and J. L. Sanz. 2005. New Trends in Reforming Technologies: from Hydrogen Industrial Plants to Multifuel Microreformers. Catalysis Reviews 47: 491–588.
- Krebs, Robert E. 1998. The History and Use of Our Earth's Chemical Elements: A Reference Guide. Westport, CT: Greenwood Press. ISBN 0313301239
- Newton, David E. 1994. The Chemical Elements. New York: Franklin Watts. ISBN 0531125017
- Rigden, John S. 2002. Hydrogen: The Essential Element. Cambridge, MA: Harvard University Press. ISBN 0531125017
- Romm, Joseph J. 2004. The Hype about Hydrogen, Fact and Fiction in the Race to Save the Climate. Washington, D.C.: Island Press. ISBN 155963703X
- Stwertka, Albert. 2002. A Guide to the Elements. New York: Oxford University Press. ISBN 0195150279
External links
All links retrieved March 29, 2014.
- “The Truth About Hydrogen” by Jeff Wise, Popular Mechanics (November 2006)
- The Legendre and Laguerre Polynomials & the Elementary Quantum Mechanical Model of the Hydrogen Atom by Timothy Jones
- Biohydrogen – Green Car Congress
- Fuel Cell and Hydrogen Energy Association
- Hydrogen Wavefunctions – HyperPhysics
- “Zinc Powder Will Drive your Hydrogen Car” – Physorg.com
- “Genetically engineered blood protein can be used to produce hydrogen gas from water” – Imperial College, London
E numbers
| ||
|---|---|---|
| Colours (E100-199) • Preservatives (E200-299) • Antioxidants & Acidity regulators (E300-399) • Thickeners, stabilisers & emulsifiers (E400-499) • pH regulators & anti-caking agents (E500-599) • Flavour enhancers (E600-699) • Miscellaneous (E900-999) • Additional chemicals (E1100-1599) | ||
| Waxes (E900-909) • Synthetic glazes (E910-919) • Improving agents (E920-929) • Packaging gases (E930-949) • Sweeteners (E950-969) • Foaming agents (E990-999) | ||
| Argon (E938) • Helium (E939) • Dichlorodifluoromethane (E940) • Nitrogen (E941) • Nitrous oxide (E942) • Butane (E943a) • Isobutane (E943b) • Propane (E944) • Oxygen (E948) • Hydrogen (E949) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.