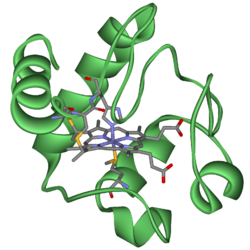
Cytochrome c
Cytochrome c, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It is a soluble protein, unlike other cytochromes, and is an essential component of the electron transfer chain, where it carries one electron. It is capable of undergoing oxidation and reduction, but does not bind oxygen. It transfers electrons between Complexes III and IV. It belongs to cytochrome c family of proteins.
Overview
Cytochromes are, in general, membrane-bound hemoproteins that contain heme groups and carry out electron transport.
A heme (American English) or haem (British English) is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic subunit; these are known as hemoproteins.
They are found either as monomeric proteins (e.g., cytochrome c) or as subunits of bigger enzymatic complexes that catalyze redox reactions. They are found in the mitochondrial inner membrane and endoplasmic reticulum of eukaryotes, in the chloroplasts of plants, in photosynthetic microorganisms, and in bacteria.
The heme group is a highly-conjugated ring system (which means its electrons are very mobile) surrounding a metal ion, which readily interconverts between the oxidation states. For many cytochromes, the metal ion present is that of iron, which interconverts between Fe2+ (reduced) and Fe3+ (oxidized) states (electron-transfer processes) or between Fe2+ (reduced) and Fe3+ (formal, oxidized) states (oxidative processes). Cytochromes are, thus, capable of performing oxidation and reduction. Because the cytochromes (as well as other complexes) are held within membranes in an organized way, the redox reactions are carried out in the proper sequence for maximum efficiency.
In the process of oxidative phosphorylation, which is the principal energy-generating process undertaken by organisms, which need oxygen to survive, other membrane-bound and -soluble complexes and cofactors are involved in the chain of redox reactions, with the additional net effect that protons (H+) are transported across the mitochondrial inner membrane. The resulting transmembrane proton gradient ([protonmotive force]) is used to generate ATP, which is the universal chemical energy currency of life. ATP is consumed to drive cellular processes that require energy (such as synthesis of macromolecules, active transport of molecules across the membrane, and assembly of flagella).
Several kinds of cytochrome exist and can be distinguished by spectroscopy, exact structure of the heme group, inhibitor sensitivity, and reduction potential.
Three types of cytochrome are distinguished by their prosthetic groups:
| Type | prosthetic group |
| Cytochrome a | heme a |
| Cytochrome b | heme b |
| Cytochrome d | tetrapyrrolic chelate of iron[1] |
The definition of cytochrome c is not defined in terms of the heme group.[2] There is no "cytochrome e," but there is a cytochrome f, which is often considered a type of cytochrome c.[3]
In mitochondria and chloroplasts, these cytochromes are often combined in electron transport and related metabolic pathways:
| Cytochromes | Combination |
| a and a3 | Cytochrome c oxidase ("Complex IV") |
| b and c1 | Coenzyme Q - cytochrome c reductase ("Complex III") |
| b6 and f | Plastoquinol—plastocyanin reductase |
A completely distinct family of cytochromes is known as the cytochrome P450 oxidases, so named for the characteristic Soret peak formed by absorbance of light at wavelengths near 450 nm when the heme iron is reduced (with sodium dithionite) and complexed to carbon monoxide. These enzymes are primarily involved in steroidogenesis and detoxification.
Cytochromes c (cytC) are electron-transfer proteins having one or several heme c groups, bound to the protein by one or, more generally, two thioether bonds involving sulphydryl groups of cysteine residues. The fifth haem iron ligand is always provided by a histidine residue. Cytochromes c possess a wide range of properties and function in a large number of different redox processes[4]. The founding member of this family is mitochondrial cytochrome c.
Ambler[5] recognized four classes of cytC. Class I includes the low-spin soluble cytC of mitochondria and bacteria, with the haem-attachment site towards the N-terminus, and the sixth ligand provided by a methionine residue about 40 residues further on towards the C-terminus. On the basis of sequence similarity, class I cytC were further subdivided into five classes, IA to IE. Class IB includes the eukaryotic mitochondrial cytC and prokaryotic 'short' cyt c2 exemplified by Rhodopila globiformis cyt c2; class IA includes 'long' cyt c2, such as Rhodospirillum rubrum cyt c2 and Aquaspirillum itersonii cytc-550, which have several extra loops by comparison with class IB cytC.
Its primary structure consists of a chain of 100 amino acids.
Variation
Cytochrome c is a highly conserved protein across the spectrum of species, found in plants, animals, and many unicellular organisms. This, along with its small size (molecular weight about 12,000 daltons), makes it useful in studies of cladistics.
The cytochrome c molecule has been studied for the glimpse it gives into evolutionary biology. Both chickens and turkeys have the identical molecule (amino acid for amino acid) within their mitochondria, whereas ducks possess molecules differing by one amino acid. Similarly, both humans and chimpanzees have the identical molecule, while rhesus monkeys possess cytochromes differing by one amino acid.
Functions
Cytochrome c can catalyze several reactions such as hydroxylation and aromatic oxidation, and shows peroxidase activity by oxidation of various electron donors such as 2,2-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), 2-keto-4-thiomethyl butyric acid and 4-aminoantipyrine.
Role in low level laser therapy
Cytochrome c is also suspected to be the functional complex in so called LLLT: Low-level laser therapy. In LLLT, laser light on the wavelength of 670 nanometer penetrates wounded and scarred tissue in order to increase cellular regeneration. Light of this wavelength appears capable of increasing activity of cytochrome c, thus increasing metabolic activity and freeing up more energy for the cells to repair the tissue.[citation needed]
Role in apoptosis
Cytochrome c is also an intermediate in apoptosis, a controlled form of cell death used to kill cells in the process of development or in response to infection or DNA damage[6] .
Cytochrome c is released by the mitochondria in response to pro-apoptotic stimuli. The sustained elevation in calcium levels precedes cyt c release from the mitochondria. The release of small amounts of cyt c leads to an interaction with the IP3 receptor (IP3R) on the endoplasmic reticulum (ER), causing ER calcium release. The overall increase in calcium triggers a massive release of cyt c, which then acts in the positive feedback loop to maintain ER calcium release through the IP3Rs. This explains how the ER calcium release can reach cytotoxic levels. This release in turn activates caspase 9, a cysteine protease. Caspase 9 can then go on to activate caspases 3 and 7, which are responsible for destroying the cell from within.
Classes
In 1991 R. P. Ambler recognized four classes of cytochrome c:
- Class I includes the lowspin soluble cytochrome c of mitochondria and bacteria. It has the heme-attachment site towards the N terminus of histidine and the sixth ligand provided by a methionine residue towards the C terminus.
- Class II includes the highspin cytochrome c'. It has the heme-mattachment site closed to the N terminus of histidine.
- Class III comprises the low redox potential multiple heme cytochromes. The heme c groups are structurally and functionally nonequivalent and present different redox potentials in the range 0 to -400 mV.
- Class IV was originally created to hold the complex proteins that have other prosthetic groups as well as heme c.
ReferencesISBN links support NWE through referral fees
- ↑ MeSH Cytochrome+d
- ↑ MeSH Cytochrome+c+Group .
- ↑ Template:EMedicineDictionary
- ↑ Moore GR, Pettigrew GW (1987). : -.
- ↑ Ambler RP (1991). Sequence variability in bacterial cytochromes c. Biochim. Biophys. Acta 1058 (1): 42-47.
- ↑ Liu X, Kim C, Yang J, Jemmerson R, Wang X (1996). Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86 (1): 147-57.
Bushnell, G.W., Louie, G.V., Brayer, G.D. (1990) High-resolution three-dimensional structure of horse heart cytochrome c. J.Mol.Biol. 214: 585-595. Retrieved May 16, 2008.
Further reading
- Skulachev VP (1998). Cytochrome c in the apoptotic and antioxidant cascades.. FEBS Lett. 423 (3): 275-80.
- Mannella CA (1998). Conformational changes in the mitochondrial channel protein, VDAC, and their functional implications.. J. Struct. Biol. 121 (2): 207-18.
- Ferri KF, Jacotot E, Blanco J, et al. (2001). Mitochondrial control of cell death induced by HIV-1-encoded proteins.. Ann. N. Y. Acad. Sci. 926: 149-64.
- Britton RS, Leicester KL, Bacon BR (2002). Iron toxicity and chelation therapy.. Int. J. Hematol. 76 (3): 219-28.
- Haider N, Narula N, Narula J (2003). Apoptosis in heart failure represents programmed cell survival, not death, of cardiomyocytes and likelihood of reverse remodeling.. J. Card. Fail. 8 (6 Suppl): S512-7.
- Castedo M, Perfettini JL, Andreau K, et al. (2004). Mitochondrial apoptosis induced by the HIV-1 envelope.. Ann. N. Y. Acad. Sci. 1010: 19-28.
- Ng S, Smith MB, Smith HT, Millett F (1977). Effect of modification of individual cytochrome c lysines on the reaction with cytochrome b5.. Biochemistry 16 (23): 4975-8.
- Lynch SR, Sherman D, Copeland RA (1992). Cytochrome c binding affects the conformation of cytochrome a in cytochrome c oxidase.. J. Biol. Chem. 267 (1): 298-302.
- Garber EA, Margoliash E (1990). Interaction of cytochrome c with cytochrome c oxidase: an understanding of the high- to low-affinity transition.. Biochim. Biophys. Acta 1015 (2): 279-87.
- Bedetti CD (1985). Immunocytochemical demonstration of cytochrome c oxidase with an immunoperoxidase method: a specific stain for mitochondria in formalin-fixed and paraffin-embedded human tissues.. J. Histochem. Cytochem. 33 (5): 446-52.
- Tanaka Y, Ashikari T, Shibano Y, et al. (1988). Construction of a human cytochrome c gene and its functional expression in Saccharomyces cerevisiae.. J. Biochem. 103 (6): 954-61.
- Evans MJ, Scarpulla RC (1989). The human somatic cytochrome c gene: two classes of processed pseudogenes demarcate a period of rapid molecular evolution.. Proc. Natl. Acad. Sci. U.S.A. 85 (24): 9625-9.
- Passon PG, Hultquist DE (1972). Soluble cytochrome b 5 reductase from human erythrocytes.. Biochim. Biophys. Acta 275 (1): 62-73.
- Dowe RJ, Vitello LB, Erman JE (1984). Sedimentation equilibrium studies on the interaction between cytochrome c and cytochrome c peroxidase.. Arch. Biochem. Biophys. 232 (2): 566-73.
- Michel B, Bosshard HR (1984). Spectroscopic analysis of the interaction between cytochrome c and cytochrome c oxidase.. J. Biol. Chem. 259 (16): 10085-91.
- Broger C, Nałecz MJ, Azzi A (1980). Interaction of cytochrome c with cytochrome bc1 complex of the mitochondrial respiratory chain.. Biochim. Biophys. Acta 592 (3): 519-27.
- Smith HT, Ahmed AJ, Millett F (1981). Electrostatic interaction of cytochrome c with cytochrome c1 and cytochrome oxidase.. J. Biol. Chem. 256 (10): 4984-90.
- Geren LM, Millett F (1981). Fluorescence energy transfer studies of the interaction between adrenodoxin and cytochrome c.. J. Biol. Chem. 256 (20): 10485-9.
- Favre B, Zolnierowicz S, Turowski P, Hemmings BA (1994). The catalytic subunit of protein phosphatase 2A is carboxyl-methylated in vivo.. J. Biol. Chem. 269 (23): 16311-7.
- Gao B, Eisenberg E, Greene L (1996). Effect of constitutive 70-kDa heat shock protein polymerization on its interaction with protein substrate.. J. Biol. Chem. 271 (28): 16792-7.
Additional images
- ETC.PNG
ETC
- Etc2.png
ETC
See also
- PEGylation
External links
- Template:UMichOPM - Calculated orientations of cytochromes c in the lipid bilayer
- MeSH Cytochrome+c
| ||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.